Answers
Answer:
9.5X10^23 molecules N2 X (2 molecules NH3 / 1 molecule N2) = 1.9X10^24 molecules NH3
Explanation:
.
Related Questions
Indicate the hybridization of each carbon atom as labeled.

Answers
Carbon atoms 1 - 7 are all sp2 hybridized carbon atoms while carbon 8 is sp3 hybridized.
What is hybridization?The term hybridization has to do with the mixing of atomic orbitals in order to obtain molecular orbitals that are of appropriate energy and can be able to participate in bonding. The idea of hybridization is rooted in the valence bond theory.
Now we have the compound as has been shown in the image attached to the question, there are carbon atoms that have been numbered from one to eight. We are to identify the hybridization pattern in each atom.
We can see that atoms 1 - 7 are all sp2 hybridized carbon atoms while carbon 8 is sp3 hybridized.
Learn more about hybridization:https://brainly.com/question/14140731
#SPJ1
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
paragraph explain how to draw a covalent bonds
Answers
Covalent bonds are those bonds in which the atoms between which the bond is present share electrons. Each element retains its electrons but shares with the element with whom it creates the bond.
To graph a covalent bond there are different structures, for example the Lewis structure, which indicates the electrons in the form of points, then the number of points of the atom will be the valence electrons that the element has. The symbol of the element is enclosed in a circle and the electrons are drawn on the circle. The electrons that are shared overlap between the circles of both elements. In the following example you can see what I just described:

What is the total pressure of a mixture that contains 50% nitrogen at 1.7 atm, 23% oxygen at 1.1 atm, 12% argon at 0.7atm, 10% methane at 0.5 atm, and 5% water vapor at 0.2 atm?A. 1.247 atmB. 4.2 atmC. 0.13 atmD. 0.85 atm
Answers
Answer:
A. 1.247 atm.
Explanation:
The Behavior of Gases => Dalton's Law of Partial Pressures.
The partial pressure of a gas is the contribution that gas makes to the total pressure when the gas is part of a mixture. Dalton's law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of all of the partial pressures of the component gases and it can be expressed as follows:
\(P_{total}=P_1+P_2+...\)In the statement, they are telling us that we have the pressures of various gases in different proportions as percentages. Remember that percentages can be written as decimals by dividing the percentages by 100: 50 % is 0.5, 23% is 0.23, and so on.
So first, let's find the partial pressures of all the gases multiplying the percentage as decimals with the given pressure:
\(\begin{gathered} P_{nitrogen}=0.5\cdot1.7\text{ atm=0.85 atm,} \\ \\ P_{oxygen}=0.23\cdot1.1\text{ atm=0.253 atm,} \\ \\ P_{argon}=0.12\cdot0.7\text{ atm=0.084 atm,} \\ \\ P_{methane}=0.1\cdot0.5\text{ }atm=0.05\text{ atm,} \\ \\ P_{water\text{ vapor}}=0.05\cdot0.2\text{ atm=0.01 atm.} \end{gathered}\)Now that we found the partial pressures, we can obtain the total pressure using the initial formula, like this:
\(\begin{gathered} P_{total}=P_{nitrogen}+P_{oxygen}+P_{argon}+P_{methane}+P_{water\text{ vapor}}, \\ \\ P_{total}=(0.85+0.253+0.084+0.05+0.01)atm, \\ \\ P_{total}=1.247\text{ atm.} \end{gathered}\)The answer would be that the total pressure is A. 1.247 atm.
which three of the following statements about autotrophs and heterotrophs on earth are true?select 3 correct answer(s)question 6 options:the majority of autotrophs on earth require aerobic respiration in order to survive.heterotrophs are dependent on autotrophs to generate o2 from h2o in order to support aerobic respiration.heterotrophs evolved on earth before autotrophs.heterotrophs depend on autotrophs for conversion of light energy to chemical energy.there are no metabolic differences between autotrophs and heterotrophs.heterotrophs that do not eat autotrophs every day will die in a short time.autotrophs depend on heterotrophs to oxidize sugar and release co2 into the atmosphere.the majority of heterotrophs on earth do not use aerobic respiration as a form of energy conversion.
Answers
Below are the three true assertions concerning autotrophs and heterotrophs on earth.
In order to support aerobic respiration, heterotrophs depend on autotrophs to produce O2 from H2O. They also depend on autotrophs to convert light energy to chemical energy. The bulk of Earth's autotrophs need aerobic respiration to survive.
Autotrophic plants use the energy from the sun and uncomplicated substances like carbon dioxide and water to store energy in food (glucose and starch). Instead of getting their energy from other living things, they get it directly from sunshine.
An organism is referred to as a heterotroph if it consumes other plants or animals for food and energy. Its origins are in the Greek words hetero, which means "other," and trophe, which means "nutrition."
To learn about autotrophs
https://brainly.com/question/11209881
#SPJ4
how many grams of Br2 are needed to completely convert 15.0g Al to Albr3?
Answers
Answer:
For, this question,
The balanced equation is; 2Al + 3 ----> 2Al
We can now, calculate how many grams of will be needed to completely convert 15 g of Al into Al.
Here, the reaction produces, 2 moles of Al
So, For 15.0 g of Al; we can calculate;
(15.0 g Al) X (1 mole Al / 26.98 g Al) X (3 moles / 2 moles Al) X (159.81 g / 1 mole )
= 133 g of will be needed.
Therefore, 133g of will be needed to completely convert 15 g of Al into Al.
Explanation:
If the charge on the chloride ion is -1, what is the charge on the magnesium ion in the compound MgCl2?
Answers
Answer:
+2
Explanation:
Because the charge of the chloride ion is negative, that means that the charge of the magnesium ion must be positive since cations and anions go together, not cation and cation nor anion and anion. Using the "reverse criss-cross method", since the subscript of Mg is 1, that means that this is the lowest whole number ratio so we don't need to worry about simplifying. Therefore, since the charge of Cl is 2, the answer is +2.
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
what is used to help water wash away greasy dirt
Answers
Answer:
soap
Explanation:soap
Answer:
A molecule with polar and nonpolar ends
Explanation:
Prepare one solution that has 0.12 M of FeCl3 and 0.40 M of HCl with the reagents 3 M HCl and Solid FeCL3 * 6H20. Provide the calculations and protocol to make the solution in a lab.
Answers
To prepare a 0.12 M solution of FeCl₃, the amount of solid FeCl₃ to be dissolved in a given volume of solvent will be 9.72 grams.
Given,
Molarity of FeCl₃ (M)= 0.12 M
The molecular weight (m) of FeCl₃ is = 162 gm
The volume of the solution (V) to be prepared is =500 ml
The amount of FeCl₃ to be dissolved to make a 0.12 M solution is= x
So,
MV= x ÷ m × 1000
0.12× 500 = x ÷ 162 × 1000
x = 60 × 162 ÷ 1000
x= 9.72 gm
So 9.72 grams of FeCl₃ is dissolved to make 500 ml of 0.12 M solution.
For preparing 0.4 M HCl from 4M HCL:
If we need to make 500 ml of solution with 0.4M of HCL, then we use the formula:
M₁V₁= M₂V₂
0.4 × 500= 4 × x
x= 50 ml
So 50 ml of 4M HCL is taken to make 0.4 M HCL.
To learn more about FeCl₃, refer to the link:
https://brainly.com/question/32098087
#SPJ1
How many grams are there in 1.4 x 1024 molecules of NH3?
Answers
2.32 atoms are there in 1.4 x 10²⁴. The smallest unit of matter with properties like chemical elements is the atom.
The atom represents the smallest portion of material that may be split without producing particles with an electrical charge. Essentially a result, an atom acts as the basic building block of chemistry. An atom is mostly made of space. The remaining material consists of a negatively charged cloud of electrons revolving about a positively charged nucleus composed of protons plus neutrons.
Number of atoms = 1.4 x 10²⁴/ 6.022×10²³
= 2.32 atoms
To know more about atom, here:
https://brainly.com/question/1566330
#SPJ1
2.What is the limiting reagent if 0.5 g Al is reacted with 3.5 g CuCl2? (Reminder: CuCl2 is a dihydrate)

Answers
3) In question 1, we find the following balanced equation:
2 Al + 3 CuCl2.2H2O ---> 3 Cu + 2 AlCl3 + 6 H2O
Step 1 - Let's find out how many moles of Al and CuCl2 reacts
For this, we will use the following formula: mole = mass/molar mass
Molar mass of Al: 26.981539 g/mol
Molar mass of CuCl2.2H2O: 147.01 g/mol
Al:
mass = 0.5 g
molar mass = 26.981539 g/mol
mole = 0.5/26.981539
mole = 0.018531189
CuCl2.2H2O:
mass: 3.5 g
Molar mass: 147.01 g/mol
mole = 0.023807904
Step 2 - Let's see which one is the limiting reactant.
2 moles Al --- 3 moles CuCl2.2H2O
0.018531189 moles Al --- x
x = 0.02779678
2 moles Al --- 3 moles CuCl2.2H2O
x moles Al --- 0.023807904 moles CuCl2.2H2O
x = 0.015871936
CuCl2.2H2O is the limiting reactant. It means that 0.023807904 moles CuCl2.2H2O react with 0.015871936 moles of Al and produce 0.023807904 moles of Cu.
Step 3 - Let's transform 0.023807904 moles of Cu into grams. For this, we use the following formula: mass = mole x molar mass
molar mass of Cu = 63.546
mole = 0.023807904 moles
mass = 0.023807904 x 63.546
mass = 1.5129 g
Answer: The theoretical yield of copper produced is 1.5129 g
Which are products of a combustion reaction?
A. CO and H20
B. Hydrocarbons and H2O
C. O2 and H2O
D. Co, and H2O
Answers
Which of the following is the correct Lewis dot structure of c4?
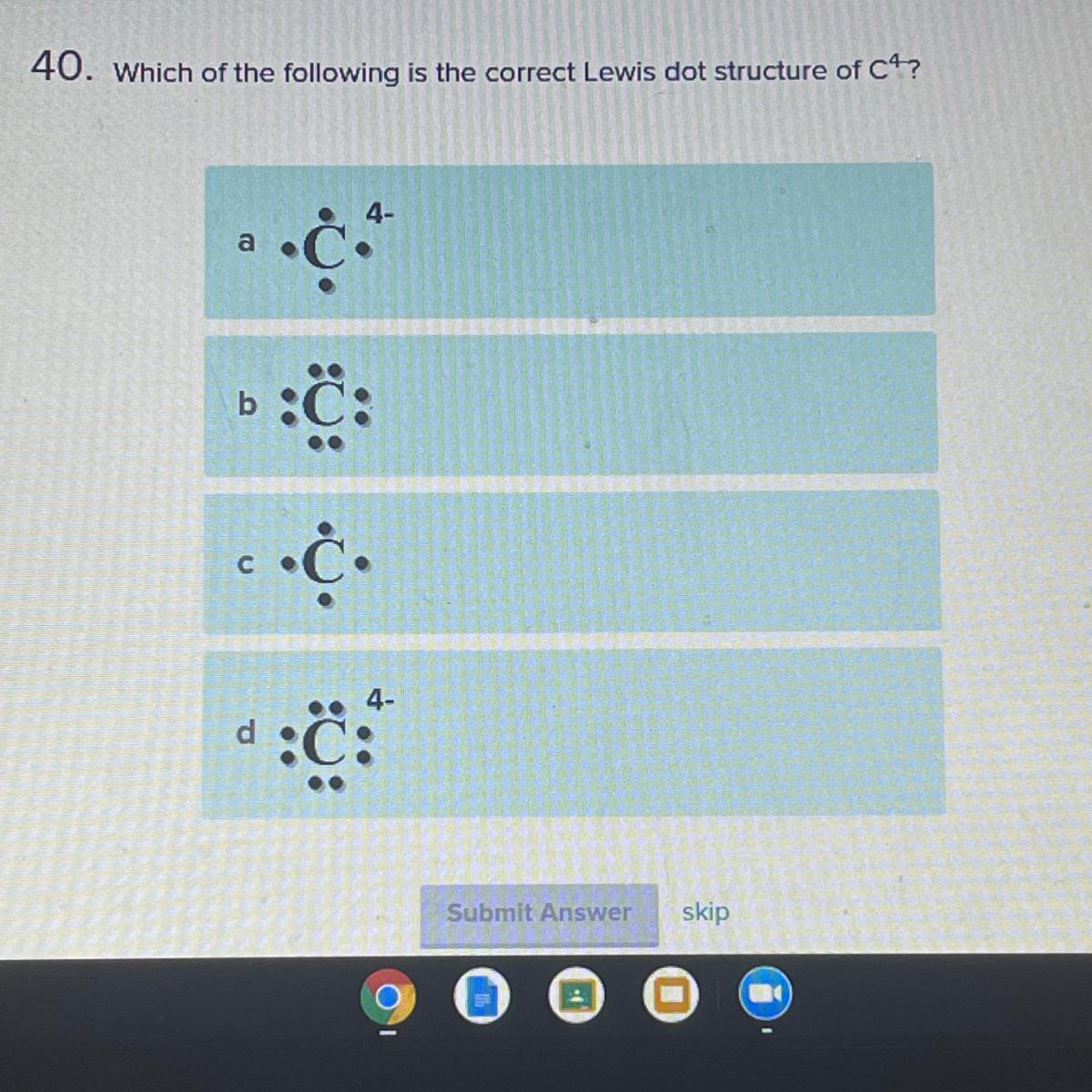
Answers
Answer: B
Explanation:
Need help with this two part question

Answers
The ideal gas law and stoichiometry must be used to calculate the volume of carbon dioxide gas produced by the breakdown of 4.09 g of calcium carbonate at STP (Standard Temperature and Pressure).
Use the molar mass of calcium carbonate (CaCO3) to determine how many moles it contains. CaCO3 has a molar mass of 100.09 g/mol.
CaCO3 mass divided by its molar mass equals the number of moles of CaCO3: 4.09 g/100.09 g/mol.
The number of moles of carbon dioxide (CO2) generated may be calculated using the stoichiometric ratio from the balancing equation. By using the equation:
A unit of CaCO3 and CO2 is produced.
CO2 moles equal the same number of moles of CaCO3.
Use the ideal gas law to translate the volume of carbon dioxide into moles.
Learn more about CaCO3 at :
https://brainly.com/question/29792038
#SPJ1
This is due tomorrow please help!

Answers
Answer:
Normal Fault -> Tension
Reverse Fault -> Compression
Strike-Slip Fault -> Shearing
Explanation:
Normal Fault -> Tension
[] A normal fault is when the walls are being pulled apart and is caused by tension
[] Example:
<- ->
Reverse Fault -> Compression
[] A reverse fault is when the walls are being pushed together and is caused by compression
[] Example:
-> <-
Strike-Slip Fault -> Shearing
[] A strike-slip fault is when the walls are being pushed apart in parallel directions and is caused by shearing
[] Example:
/\ \/
Note: The page you provided also gives the answers for this specific question.
Have a nice day!
I hope this is what you are looking for, but if not - comment! I will edit and update my answer accordingly. (ノ^∇^)
- Heather
If you dilute a 10.0 mL of a 11.5 M HCl solution to make a 1.10 M HCl solution, what will the final
volume be?
Answers
Explanation:
The final volume will be 100 mL. This is because when you dilute a solution, the molarity of the solution decreases while the volume increases. Since the molarity of the original solution is 11.5 M and the desired molarity is 1.10 M, the volume must increase by a factor of 10.5 (11.5/1.10). Therefore, the final volume will be 10.0 mL x 10.5 = 100 mL.
Classify the molecules based on whether the molecule has a standard enthalpy of formation, AH;, equal to 0. Assume all
conditions are at standard pressure and temperature (STP).
Answers
The standard enthalpy of formation of substance in its standard state is zero.
Enthalpy is defined as a thermodynamic quantity that describes the energy of a system. For substances in their standard state, the enthalpy of formation is zero.
The standard state of a substance is defined as the state in which it is found under standard conditions. The following substances has their standard ethalpy of formation as zero or not zero;
Zero enthalpy of formation Non zero enthalpy of formation
Cl2(g) I2(s)
Br(g) Br2(l)
I2(g) Br2(s)
Hg(l) Hg(s)
Learn more: https://brainly.com/question/13164006

it's science pls help

Answers
Answer:
1.5
Explanation:
Ba(ClO 4 ) 2 (aq)+K 2 SO 4 (aq) -> BaSO 4 (s)+2 KClO 4 (aq)
Write the complete ionic equation
Answers
Answer:
sorry di ko alam
Explanation:
What is the molecule shown below?н син н Н НТТТТТТн-с-с-с-с-с-с-н||||||H H CHOH Н НO A. OctaneOB. 4-propylpentaneOC. 2,3-dimethylhexaneOD. 2-pentylpropane

Answers
This molecule in the question presents 6 Carbons in its main chain, counting only the carbons in the middle, which makes it a Hexane molecule. Besides the main chain carbons, we also have two methyl groups linked to carbons 2 and 3, counting from left to right. Therefore the final name will be:
2,3-dimethylhexane. Letter C
What is the steps of the photosynthetic equation?
Answers
Answer: The photosynthesis equation is as follows: 6CO2 + 6H20 + (energy) → C6H12O6 + 6O2 Carbon dioxide + water + energy from light produces glucose and oxygen.
Explanation: Photosynthesis is comprised of two stages, the light-dependent reaction and the light-independent reactions, so that for the light suports energy.
Hope this helps:)
If a solution has a temperature of 55 K, what is its temperature in degrees Celsius? A) 131°C B) 328°C C) 12.8°C D) 155°C E) -218°C
Answers
Answer:
E. -218°C
Explanation:
hope it help you (o´・Υ・)ノ・
If a solution has a temperature of 55 K, -218°C is its temperature in degrees Celsius. The correct option is option E.
What is temperature?The physical concept of temperature indicates in numerical form how hot or cold something is. A thermometer is used to determine temperature.
Thermometers are calibrated using a variety of temperature scales, which historically defined distinct reference points as well as thermometric substances. The most popular scales are the Kelvin scale (K), which is mostly used for scientific reasons, this same Fahrenheit scale (°F), as well as the Celsius scale, which has the unit sign °C.
°C + 273 += K
°C = 55 K- 273= -218°C
55 K = -218°C
Therefore, if a solution has a temperature of 55 K, -218°C is its temperature in degrees Celsius. The correct option is option E.
To learn more about temperature, here:
https://brainly.com/question/23411503
#SPJ2
Which of the following statements are true concerning the results of the Human Genome Project? Check all that apply.
Researchers now have maps of every human’s genome.
The Human Genome Project has raised many complicated ethical issues.
All medical conditions can be attributed to a specific gene.
Researchers now have a map of an “average” human genome.
Answers
The following statement is true concerning the results of the Human Genome Project:
The Human Genome Project has raised many complicated ethical issues.
What is Human Genome?
The human genome refers to the complete set of genetic information (DNA) present in human cells. It includes both the protein-coding and non-coding regions of DNA. The human genome is made up of about 3 billion base pairs and is organized into 23 pairs of chromosomes. The study of the human genome is an important field of genetics and has many implications for understanding human biology, evolution, and disease.
The Human Genome Project was a scientific research project that aimed to identify and map all the genes in the human genome, which contains all the genetic information needed for human development and function. The project was completed in 2003 and provided a wealth of information about the structure and function of the human genome, including the location and sequence of all human genes.
Learn more about Human Genome from the given https://brainly.com/question/29479722
#SPJ1
How is steel made from the raw product of the blast furnace known
as "pig iron"? What are the advantages of using steel?
List references used (if any were used) to answer this question.
Answers
Steel is produced from pig iron through a process known as steelmaking or iron and steel production.
The pig iron obtained from the blast furnace contains high amounts of carbon, impurities, and other elements. To convert pig iron into steel, the carbon content needs to be reduced to desired levels, and impurities must be removed.One common method of steelmaking is the basic oxygen process (BOP). In this process, pig iron is placed in a vessel called a converter, where oxygen is blown through the molten metal. The oxygen reacts with the carbon and impurities, causing them to oxidize and form gases that are released. Alloying elements and desired additives can be added at this stage to achieve specific steel properties. Another method is the electric arc furnace (EAF), where an electric arc is used to heat and melt the pig iron, allowing impurities to be oxidized and removed.The advantages of using steel are numerous. Steel is strong, durable, and versatile, making it suitable for a wide range of applications. It has high tensile strength, which means it can withstand heavy loads and pressures. Steel is also resistant to corrosion, making it ideal for construction, infrastructure, and transportation projects. It is a recyclable material, contributing to sustainability and reducing environmental impact. Additionally, steel can be fabricated into various shapes and sizes, allowing for customization and flexibility in design.References:
A. Ghosh and A. Chatterjee, Ironmaking and Steelmaking: Theory and Practice, PHI Learning, 2008.
R.H. Tupkary and V.R. Tupkary, An Introduction to Modern Iron Making, Khanna Publishers, 2010.
J.R. Davis, ed., ASM Specialty Handbook: Carbon and Alloy Steels, ASM International, 1995.
for such more questions on production
https://brainly.com/question/25597694
#SPJ8
What mass of sodium hydroxide, NaOH, would be required to produce 16 g of the antacid milk of magnesia [magnesium hydroxide, Mg(OH)2] by the following reaction? MgCl2(aq) + 2NaOH(aq) ⟶ Mg(OH)2(s) + 2NaCl(aq)
Answers
It would take 22g of sodium hydroxide (NaOH) to make 16g of the antacid milk of magnesia (magnesium hydroxide).
Simply put, what is stoichiometry?In the field of chemistry known as stoichiometry, desired quantitative data is ascertained by using relationships between the reactants and/or products of a chemical reaction. Stoichiometry literally translates as the measure of elements because the Greek words stoikhein and metron both mean element and measure, respectively.
What is the stoichiometric law?In a chemical reaction, the total mass of reactant and product are equal, according to the statement, and neither is generated nor destroyed. This is the stoichiometric law, and also the law of conservation of mass.
\(16 \mathrm{~g} \text { of } \mathrm{Mg}(\mathrm{OH})_2 \times \frac{1 \mathrm{molMg}(\mathrm{OH})_2}{58.3 g \mathrm{gg}(\mathrm{OH})_2} \times \frac{2 \mathrm{~mol} \mathrm{NaOH}}{1 \mathrm{molMg}(\mathrm{OH})_2} \times \frac{40 \mathrm{gNaOH}}{\mathrm{molNaOH}}=22 \mathrm{~g}\)
To learn more about Stoichiometry visit:
brainly.com/question/29775083
#SPJ1
Consider Example 12.10. Suppose the experiment is repeated with 0.032 mol of helium instead with everything else staying the same, what is the amount of heat required now to achieve that process
Answers
Answer:
120 extracted from the gas
Explanation:
The amount of heat required now to achieve that process is 60 J of heat added to the gas.
Heat required to achieve the processThe amount of heat required to achieve the process is calculated as follows;
\(E = \frac{3}{2} nR\Delta T\)
where;
n is number of moles of the helium gas = 0.032 moleR is ideal gas constant = 8.314 J/mol.KΔT is change in temperature (from example 12.10) = 450 K - 300 K = 150 K\(E = \frac{3}{2} \times 0.032 \times 8.314 \times (150)\\\\E = 60 \ J\)
Thus, the amount of heat required now to achieve that process is 60 J of heat added to the gas.
Learn more about amount of heat here: https://brainly.com/question/13439286
#SPJ2
How many moles of NaHCO3 are in 2.4 x
1024 molecules of NaHCO:?
Answers
4.0 moles NaHCO
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
MolesAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
[Given] 2.4 × 10²⁴ molecules NaHCO
[Solve] moles NaHCO
Step 2: Identify Conversion
Avogadro's Number
Step 3: Convert
[DA] Set up: \(\displaystyle 2.4 \cdot 10^{24} \ molecules \ NaHCO(\frac{1 \ mol \ NaHCO}{6.022 \cdot 10^{23} \ molecules \ NaHCO})\)[DA] Divide [Cancel out units]: \(\displaystyle 3.98539 \ moles \ NaHCO\)Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
3.98539 moles NaHCO ≈ 4.0 moles NaHCO
Consider the orbital diagram shown. Which electron rule is broken in the diagram?
A. Aufbau Principle
B. Hund's Rule
C. Pauli Exclusion Principle

Answers
According to the provided orbital diagram, Hund's Rule looks to be the electron rule that is broken. According to Hund's rule, when degenerate orbitals with equal energy are accessible, electrons will first fill them individually before doing so in pairs.
While it should have been singly occupied in a distinct 2p orbital before partnering up, the second electron in the 2p orbital in the following diagram is paired with the first electron. Therefore, B. Hund's Rule is the appropriate response.
Hund's ruleThe way electrons are inserted into subshells of an atom is determined by the quantum mechanical concept known as Hund's rule. According to this theory, electrons will first occupy each orbital individually with their spins parallel (having the same spin quantum number) before teaming up with electrons in other orbitals when many orbitals with the same energy (degenerate orbitals) are available.This means that the first electron will occupy one of the available three degenerate 2p orbitals, for instance, and the second electron will occupy a different orbital with the same spin. The electrons won't start pairing up until all three orbitals are fully occupied.learn more about Hund's rule here
https://brainly.com/question/2104472
#SPJ1
Which material is the best conductor of thermal energy? O A. Metal O B. Rubber O C. Glass O D. Plastic