explain why numerical prefixes are not needed on the names of ionic compounds ?
Answers
Answer:
two ions can combine in only one combination.The number of ions is not fixed in a compound.
Related Questions
a solution of 0.2 m boric acid is prepared as an eye wash. what is the approximate ph of this solution? for boric acid ka
Answers
The approximate pH of a 0.2 M solution of boric acid as an eye wash is around 5.14.
To understand how the pH is calculated for a solution of boric acid, it's helpful to have a basic understanding of acid-base chemistry. When an acid is dissolved in water, it donates a hydrogen ion (H+) to the water molecules, forming hydronium ions (H3O+). The more hydrogen ions present in the solution, the lower the pH (since pH is a measure of the concentration of hydrogen ions).
Boric acid (H3BO3) is a weak acid, which means it only partially dissociates in water. It donates a hydrogen ion to form the conjugate base (H2BO3^-), but some of the molecules remain undissociated. The acid dissociation constant (Ka) is a measure of how much of the acid dissociates, and is calculated by dividing the concentration of the conjugate base by the concentration of the acid.
For boric acid, Ka is 5.8 x 10^-10. This is a very small number, which means the acid is not very strong. To calculate the pH of a 0.2 M solution of boric acid, we use the formula:
pH = (1/2) x (-log(Ka) + log([HA]))
where [HA] is the concentration of the acid (0.2 M). The factor of 1/2 is because boric acid donates two protons (H+) when it dissociates, but the dissociation is incomplete, so we only count half of the protons.
Plugging in the values, we get:
pH = (1/2) x (-log(5.8 x 10^-10) + log(0.2)) = 5.14
So the pH of a 0.2 M solution of boric acid as an eye wash is approximately 5.14. This is slightly acidic, but still within the safe range for eye wash solutions.
To learn more about pH of solution visit:
brainly.com/question/30934747
#SPJ11
What are the trends for atomic size and ionization? Explain why for each trend. Tell me the direction- up or down a group and across a period for each trend. Use the lecture, the video at the end of the lecture, or the guide for periodic trends to respond to this prompt.
Answers
Answer:
See explanation
Explanation:
Atomic size increases down the group due to the addition of more shells.
As more shells are added and repulsion of inner electrons become more significant, atomic size increases down the group. However, across the period, atomic size decreases due to increase in effective nuclear charge without any increase in the number of shells. This causes increased attraction between the nucleus and the outermost shell thereby decreasing the size of the atom.
Ionization energy decreases down the group because the outermost electron is more shielded by inner electrons making it easier for this outermost electron to be lost. Across the period, ionization energy increases due to increase in effective nuclear charge which makes it more difficult to remove the outermost electron due to increased nuclear attraction.
With a dialysis bag that is impermeable to sucrose, a mass change of a beaker (with 0.1M sucrose) containing a dialysis tubing with 0.8M sucrose is due to
Answers
The mass change of the beaker containing a dialysis tubing with 0.8M sucrose, enclosed by a dialysis bag impermeable to sucrose, is due to the movement of water molecules through the dialysis bag.
The sucrose concentration inside the dialysis tubing is higher (0.8M) compared to the sucrose concentration in the beaker (0.1M). This creates an osmotic gradient, where water molecules tend to move from an area of lower solute concentration (the beaker) to an area of higher solute concentration (inside the dialysis tubing).
As water molecules move across the dialysis bag, the beaker experiences a net loss of water, resulting in a decrease in mass. The sucrose molecules, being too large to pass through the impermeable dialysis bag, remain inside the tubing.
This process, known as osmosis, continues until the sucrose concentrations inside and outside the dialysis tubing reach equilibrium. At this point, the mass change of the beaker will stabilize, and no further net movement of water will occur.
To know more about osmosis , refer here:
https://brainly.com/question/31028904#
#SPJ11
18. In order to make one molecule of glucose, how many carbon dioxide, ATPs, and NADPH are required?
Answers
To produce one molecule of glucose, 6 molecules of carbon dioxide (\(CO_{2}\)), 18 molecules of adenosine triphosphate (ATP), and 12 molecules of nicotinamide adenine dinucleotide phosphate (NADPH) are required.
Glucose, a six-carbon sugar, is synthesized through the process of photosynthesis in plants. It involves the Calvin cycle, which incorporates carbon dioxide, ATP, and NADPH to produce glucose. For each molecule of glucose formed, 6 molecules of carbon dioxide are required.
The energy needed for glucose synthesis is provided by ATP, which is an energy-rich molecule. In the Calvin cycle, the synthesis of one glucose molecule requires 18 molecules of ATP.
NADPH, a coenzyme involved in energy transfer reactions, is required for the reduction of carbon dioxide during the Calvin cycle. In the process, 12 molecules of NADPH are utilized to produce one molecule of glucose. These components play crucial roles in capturing and storing energy, as well as providing carbon atoms for the formation of glucose, which serves as a vital energy source for organisms.
Learn more about Calvin cycle here:
https://brainly.com/question/26846190
#SPJ11
What is the mass percent when 66 grams of NaCl is dissolved in 255 grams of water?
Answers
INFORMATION:
We have:
- 66 grams of NaCl
- 255 grams of water
If the NaCl is dissolved into the water, we must find the mass percent
STEP BY STEP EXPLANATION:
To find the mass percent we must must use the next formula
\(\text{ mass percent}=\frac{\text{ mass of solute}}{\text{ mass of solution}}\times100\)In our case,
- the solute is NaCl
- the solution would be the sum of masses from NaCl and water
Then,
- mass of solute = 66 g
- mass of solution = 66 g + 255 g = 321 g
Finally, replacing in the formula,
\(\text{ mass percent}=\frac{66g}{321g}\times100=20.5607\)ANSWER:
The mass percent when 66 grams of NaCl is dissolved in 255 grams of water is 20.60%
PLEASE ANSWER ASAP (WILL GIVE BRAINLIEST)
What do we call the invisible force exerted by magnets?
A. Magnetic force
B. Electromagnetic force
C. Electric force
D. Applied force
Answers
Answer:
Magnetic Forceeeeeeeeeee
Consider the following species when answering the following questions:
(i) PCl3 (ii) CCl4 (iii) TeCl4 (iv) XeF4 (v) SF6
For which of the molecules is the molecular geometry (shape) the same as the VSEPR electron domain arrangement (electron domain geometry)?
Answers
The molecular geometry (form) of CCl4 SF6 is identical to the configuration of the electron domains in a VSEPR.
Is VSEPR and molecular geometry equivalent?VSEPR distinguishes between molecular geometry, which defines how the atoms in a molecule are ordered, and electron group geometry, which expresses how electron groups (bonds and nonbonding electron pairs) are arranged.
What do electron domains and molecule structure reveal from VSEPR?Chemistry frequently employs the valence-shell electron-pair repulsion (VSEPR) model to foretell the three-dimensional organization, or geometry, of molecules. This model accounts for the repulsion between electron pair to estimate the structure of a molecule.
Learn more about VSEPR here:
https://brainly.com/question/28775578
#SPJ4
What's the fomular for this?

Answers
Answer:
1000 km/s is the formula for density of liquid
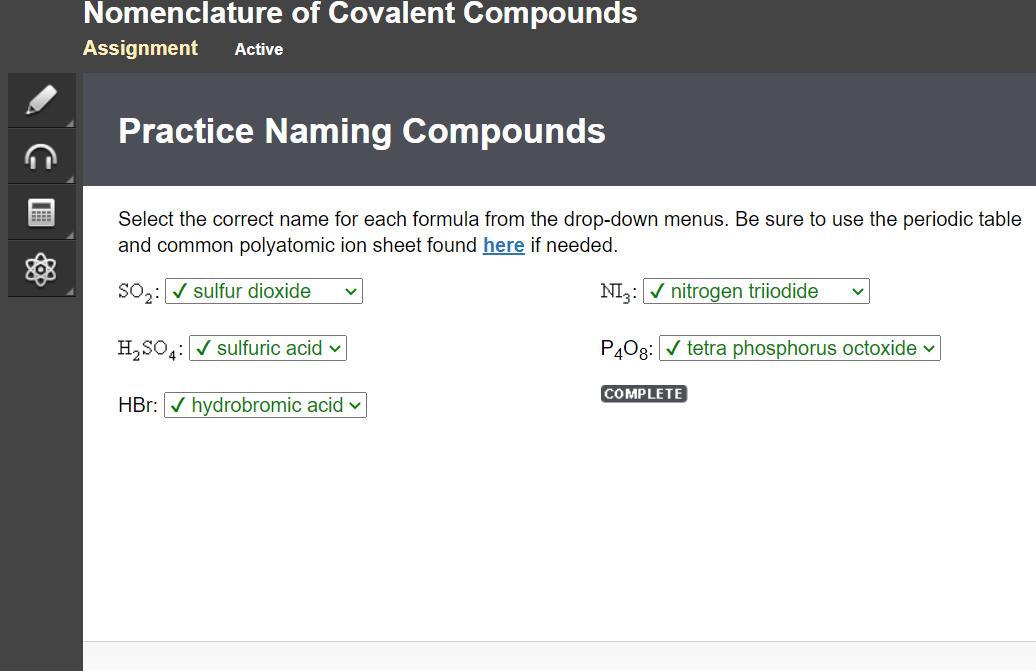
Select the correct name for each formula from the drop-down menus. Be sure to use the periodic table
and common polyatomic ion sheet found here if needed.
Answers
Answer:
here are the answers edge2020
Explanation:

Answer:
Here is 2/5 and the next slide answers too!
Explanation:
Hope it helps!!

A sample of an unknown substance has a mass of 0. 465 kg. If 3,000. 0 j of heat is required to heat the substance from 50. 0°c to 100. 0°c, what is the specific heat of the substance?.
Answers
The specific heat of an unknown substance is calculated as follows:First, calculate the heat energy required to increase the temperature of the unknown substance from 50.0°C to 100.0°C by using the formula:Q = mcΔTwhere Q is the heat energy, m is the mass, c is the specific heat, and ΔT is the change in temperature.
Substituting the values we have:3000.0 J = (0.465 kg) c (100.0°C - 50.0°C)Simplifying the equation:3000.0 J = 0.465 kg c (50.0°C)Dividing both sides by (0.465 kg) (50.0°C):c = 3000.0 J / (0.465 kg × 50.0°C)c = 128.21 J/(kg°C)Therefore, the specific heat of the unknown substance is 128.21 J/(kg°C).
The specific heat of the unknown substance is 128.21 J/(kg°C). It is a general understanding that the temperature of an object rises as heat is added to it. This is described by the equation ΔQ = mcΔT, which relates the quantity of heat transferred to an object with its mass, specific heat capacity, and change in temperature.
To know more about substance visit:
https://brainly.com/question/13320535
#SPJ11
Which of these is an example of a hypothesis?
Add different kinds of soil to three potted plants.
Observe fish swimming up a stream.
Temperature changes may be due to wind patterns.
Wonder why some layers of rocks contain fossils.
Answers
Explanation: because it “wonders”
When mitosis occurs, what is the starting product and the final product(s)?
Answers
Answer:
Image result for When mitosis occurs, what is the starting product and the final product(s)?
The result of mitosis is two identical daughter cells, genetically identical to the original cell, all having 2N chromosomes.Explanation:
Mitosis is a stage of cell division where the initial product is a parent nuclei and final products two identical daughter cells.
What is mitosis?Mitosis is a method of replicating chromosomes where they are divided into two new nuclei. Mitosis, the process of cell division, creates genetically identical cells with a constant number of chromosomes..
Telophase and cytokinesis, which splits a cell's cytoplasm, organelles, and cell membrane into two new cells with nearly equal amounts of these biological components, frequently follow.
The division of the mother cell into two genetically identical daughter cells, or the mitotic (M) phase of an animal cell cycle, is defined by the several stages of mitosis taken collectively.
Two daughter nuclei with the same genetic makeup are the outcome.of mitosis.The remainder of the cell could then carry out further cytokinesis division to create two daughter cells.
To find more about mitosis, refer the link below:
https://brainly.com/question/26678449
#SPJ2
Name organs and is the tissues found in them.
Answers
Answer:
epithelial tissue, connective tissue, muscle tissue, and nervous tissue.
I think its right
brainiest pls
Explanation:
As the temperature of air is reduced to its dew point, which of these is most likely to occur? group of answer choices freezing condensation melting supercooling evaporation
Answers
Condensation is also more likely to occur when the temperature drops to the dew point.
What is condensation?Condensation is the process of turning a gas or vapor into a liquid. This happens when gas molecules come into contact with a cold surface and attract each other, creating tiny droplets of liquid. In nature, condensation is observed when water vapor in the air forms clouds and then rains. It can also be seen when water vapor from boiling water or steam forms droplets on the outside of a cold glass or spoon. Condensation is an important process in the water cycle and essential for fresh water production.
To learn more about condensation, visit:
https://brainly.com/question/1447093
#SPJ4
Lithium is not nearly as abundant as sodium. If sodium ion batteries that function as lithium ion ones were developed, do you think sodium cobalt oxide would still work as the electrode material?
Answers
Answer:
No, sodium cobalt oxide would not work as the electrode material
Explanation:
Sodium is a much larger cation than lithium. Remember that ion size is a very important consideration when developing a battery. The ions to be substituted must be nearly of the same size if one ion is to replace the other in a crystal.
Since sodium ions are larger than lithium ions, sodium cobalt oxide would not work as the electrode material in Lithium ion batteries.
A student is reading the volume from a standard 50-mL buret identical to what you used in the lab. The bottom of the meniscus is exactly on the 20-mL line. What should she record as the correct volume
Answers
Answer:
20 mL
Explanation:
The student should record 20 mL as the correct volume.
The curved surface of a liquid that is usually observed in a buret is referred to as the meniscus. This meniscus is created as a result of the surface tension of the liquid against the walls of the buret. Hence, in order to avoid errors due to parallax, the bottom of the meniscus should be read and not the top.
Therefore, the correct volume that the student should record is 20 mL.
I will need help plz thank you
The Earth's surface is _____ water.
50 %
71 %
97 %
Answers
Answer:
71%
Explanation:
What is the mass of aluminium of 306 g of aluminium oxide
Answers
Answer:
Tha nolar mass of aluminium is 26.98 g/mol; the molar mass of (atomic) oxygen is 16.90 g/mol.
Explanation:
1 mol of Al2O3 therefore has a mass of 2(26.98)+3(16.00)=101.96 g.
Divide this into the given mass, to find the number of moles here:
(204 g)/(101.96 g/mol)=2.001 mol
There are 2 moles of aluminium per mole of aluminium oxide, so there are 2(2.001)=4.002 moles of aluminium in the sample.
Then there are (4.002 mol)(26.98 g/mol)=107.97 g of Al. Since the given mass has only 3 significant figures, round this answer o 108 g.
classify the following chemical reaction
2Li + Cu(No3)2 → Cu + 2 LiNO3
Answers
Answer:
im on this question i need help with it as well lol
Explanation:
I need an explanation on how to balance equations for mole ratio

Answers
Answer:
12. 5
11.
10
10
15. 7
14. 11
2
=
16
16
18. 3
+
11
17
3
4
co
loo
8
Step-by-step explanation:
12. 5
11.
10
10
15. 7
14. 11
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
Is this statement true or false? this experiment used pennies that were minted after 1982. These pennies are different because they contain more copper than pre-1982 pennies.
Answers
Answer:
False
Explanation:
Newer pennies contain less copper and more zinc
How many atoms are there in a 2 moles of oxygen atoms?
Answers
Explanation:
Solution — 1 molecule of O2 = 2 oxygen atoms So, 1 mole of O2 = 2 mole oxygen atoms = 2 × 6.022 × 1023 = 12.044 ×1023 oxygen atoms.
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 1.2044 ×10²⁴ oxygen atoms are there in a 2 moles of oxygen atoms.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Mathematically,
number of mole of O\(_2\)= 2 mole
number of atoms/molecules=number of moles × 6.022×10²³(Avogadro number)
substituting all the given values in the above equation, we get
number of oxygen atoms = 2 × 6.022 × 10²³
number of oxygen atoms= 1.2044 ×10²⁴ oxygen atoms.
Therefore, 1.2044 ×10²⁴ oxygen atoms are there in a 2 moles of oxygen atoms.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ2
Approximately how long will it take for 75 percent of the initial amount of C25H30N3. +(aq) to react?
A. 75 sec.
B. 225 sec.
C. 300 sec.
D. 600 sec
Answers
Approximately 225 sec, it will take for 75 percent of the initial amount of C\(_{25}\)H\(_3\)ON\(_3\)⁺(aq) to react. Therefore, the correct option is option B.
What is absorbance?The logarithm of a ratio of incident over transmitted radiant power throughout a sample is known as absorbance. As an alternative, absorbance can be characterized simply "the negative logarithm with one less absorptance, as determined on a homogeneous sample," for samples that scatter light.
In several technical fields, the phrase is used to describe how experimental measurement data are quantified. Although the phrase refers to measuring how much light is absorbed, it is frequently confused with measuring how much light is "lost" to either a detector system by other methods. Approximately 225 sec, it will take for 75 percent of the initial amount of C\(_{25}\)H\(_3\)ON\(_3\)⁺(aq) to react.
Therefore, the correct option is option B.
To know more about absorbance, here:
https://brainly.com/question/23938376
#SPJ2
Your question is incomplete but most probably your full question was,
Approximately how long will it take for 75 percent of the initial amount of C25H30N3. +(aq) to react?
A. 75 sec.
B. 225 sec.
C. 300 sec.
D. 600 sec

What do we call the sugar in
sweet tea?
A. solute
B. solvent
C. solution
Answers
Answer:
A. solute
Hope this helps
Pls big test What are openings in the ground that release energy from deep inside the planet? * 1clouds 2volcanoes 3sun 4hurricanes
Answers
Answer:
2. Volcanoes
Explanation:
The interior of some planets (e.g earth) contains some hot flowing fluids called lava which has a very high thermal energy. The lava as a means of escaping the confinement within the planet, burst out by creating an opening through the ground to the surface of the planet to release the energy (lava).
This process in which the energy is released to the surface of the planet is termed volcanic eruptions. Which occurs majorly in some susceptible regions of the earth.
The openings in the ground that release energy from deep inside the planet is thus called volcanoes.
What are 3 ways you know something is living
Answers
4. Calculate how many moles of CO, could theoretically be produced when 10 g of glucose reacts. Show your work.
5. Calculate the number of moles of carbon dioxide actually produced. The experiment is conducted at a temperature of 318 K and a pressure of 1.0 atm. The student estimates the volume of the balloon to be 550 mL (0.55 L) after the reaction takes place.
Show your work.
6. Did all the glucose react during the experiment? Justify your answer.
7. If the student's estimate of the balloon's volume was incorrect and the actual volume was 620 mL, would the amount of glucose that actually reacted be more than or less than the amount calculated above? Explain your response.

Answers
Glucose concentration causes yeast to produce more fermentation, but when the saturation gradient is reached, carbon dioxide production stops.
A reaction is what in chemistry?Chemical processes happen when atoms create or dissolve chemical bonds. Products are the molecules that are produced as a result of a chemical reaction, whereas reactants are the ingredients that initiate one.
What is response, for instance?A chemical reaction happens when one or more substances change into one or more new substances. Consider the rust that results from the reaction between iron and oxygen. When vinegar & baking soda are mixed, sodium acetate, nitrous oxide, or water are the results.
To know more about Reaction visit:
brainly.com/question/28984750
#SPJ1
Provide an acidic or basic environment to optimize digestion______(Chemicals)
Answers
Provide an acidic or basic environment to optimize digestion hydrochloric acid (HCl).
Food is broken down into more digestible, smaller pieces throughout the digestion process so that the body can absorb and use them.
The digestive system's secretions produce the acidic or basic environment necessary for effective digesting. In order to create a highly acidic environment, the stomach secretes hydrochloric acid (HCl), which lowers the pH of the stomach.
The small intestine, on the other hand, is kept at a pH that is somewhat basic to enhance the activity of the digestive enzymes that break down carbs, proteins, and lipids.
To know more about digestion, here
brainly.com/question/29030031
#SPJ4
In an experiment, a solution required 30. 05 g of nacl, 50. 0 g of , and 0. 4006 g of mgso4. Using the correct number of significant figures, what is the resulting mass?.
Answers
Using significant figures and rounding up, the resulting mass is 80.5 g.
When using addition or subtraction, the total number of significant figures is relevant. Instead, the last significant figure of every number is considered.
In 30.05, the last significant figure is 5, and it is in the hundredths. In 50.0, the last significant figure is 0, and it is in the tenths. Finally, in 0.4006, the last significant figure is 6, and it is in the ten thousandths. Of the three, the 0 from 50.0 is in the "highest" position, and so the last significant figure of the results should also be in the tenths.
If we add the numbers up, we get:
30.05 g + 50.0 g + 0.4006 = 80.4506 g
Because the last significant figure should be in the tenths, we are going to round up 4 to 5, because trailing numbers are greater than 0, so the final mass will be 80.5 g.
You can learn more about significant figures here:
brainly.com/question/17396594
#SPJ4