Answers
Answer:
zn + Hcl cual es su rreaccion
Related Questions
CxHy +O2 --> H2O + CO2
Question 1 options:
Decomposition
Combustion
Synthesis
Single Displacement
Look down below at the picture thank you

Answers
Single displacement because the oxygen is not balance
I NEED THIS RIGHT NOW!! Daria had some sand from the beach. The mass of the sand was 72 grams. She used the graduated cylinder below to measure the volume.
What is the volume of the sand found in the graduated cylinder? _____ mL

Answers
Daria had some beach sand with her. The sand has a 72 gramme mass. She calculated the volume using the graduated cylinder below. The graduated cylinder contains 15 mL of sand.
The volume of the sand is calculated using the graduated cylinder below. The sand's bulk is specified as 72 grammes.
We can use the water displacement method to calculate the volume of the sand. Following is a description of how to estimate the amount of sand using the water displacement method:
The graduated cylinder of water should first be measured for volume.
The graduated cylinder's water volume should then be measured after adding the sand to it. The volume of water increases by the same amount.
Let's use the provided problem to implement this approach.
In the beginning, there is 10 mL of water in the graduated cylinder. The graduated cylinder contains 25 mL of water once the sand has been added.
The amount of sand is therefore equal to the difference between the two volumes, which is: Sand volume equals final water volume minus initial water volume (25 - 10 = 15 mL).
As a result, there are 15 mL of sand in the graduated cylinder.
Answer : 15
For more such questions on volume, click on:
https://brainly.com/question/14197390
#SPJ8
4- The standard potential of cell: Sn/Sn²+||Cr³+/Cr is −0.60V.what is the standard
reduction potential of the Cr³+/Crelectrode? Es = -0.14V
Sn²+
(b) +0.74V
(c) -0.88V
(d) -0.74V
(a) +0.88V
Answers
The standard reduction potential of the Cr³+/Cr electrode is -0.46V. None of the option is correct.
To determine the standard reduction potential of the Cr³+/Cr electrode, we can use the Nernst equation, which relates the standard reduction potential to the cell potential under non-standard conditions. The Nernst equation is given by:
E = E° - (0.0592/n) * log(Q)
where E is the cell potential, E° is the standard reduction potential, n is the number of electrons transferred in the half-reaction, and Q is the reaction quotient.
In this case, we have the standard potential of the cell as −0.60V. We know that the standard reduction potential of the Sn/Sn²+ electrode is -0.14V. Therefore, the reduction potential of the Cr³+/Cr electrode can be calculated as:
E = -0.60V - (-0.14V)
E = -0.60V + 0.14V
E = -0.46V
Therefore, the standard reduction potential of the Cr³+/Cr electrode is -0.46V.
For more such question on standard reduction potential visit;
https://brainly.com/question/31482299
#SPJ8
what are the top three sources of the U.S electricity generation?
Answers
Answer: The three major categories of energy for electricity generation are fossil fuels (coal, natural gas, and petroleum), nuclear energy, and renewable energy sources
Explanation:
a. The sand and water together in the beaker is called a
Answers
Answer:
sand and water together in the beaker is called a mixture
Status: Not yet answered | Points possible: 1.00
A sample of chlorine gas starting at 681 mm Hg is placed under a pressure of 992 mm Hg and reduced to a volume of 543.8 mL.
What was the initial volume, in ml, of the chlorine gas container if the process was performed at constant temperature?
Type answer:
Answers
Answer:
V1 = 792.1 ml
Explanation:
The product of pressure and volume is constant when temperature is constant. This relationship is known as Boyle's law.
To answer this, I assume the chlorine gas will behave as a perfect gas. In reality this is not completely true.
P1*V1 = P2*V2
Given:
P1 = 681 mm Hg
V1 = ?
P2 = 992 mm Hg
V2 = 543.8 ml
V1 = ( P2 * V2 ) / P1
V1 = (992 *543.8 ) / 681
V1 = 792.143318649046 ml
V1 = 792.1 ml
1.What happens when non-metals react with oxygen? (1 Point)
a) Metal oxides are formed.
b) Basic oxides are formed.
c) Acidic oxides are formed
Answers
Answer:
c)
Explanation:
acidic oxides are formed
metal oxides and basic oxides are basically the same.
what energy transformations does microwave have
Answers
Answer:
radiant energy
Explanation:
microwaves convert electrical energy into radiant energy
Which type(s) of matter would need to be separated by chemical methods?
Answers
Answer:
solids, liquids, gases, or plasma
Explanation:
In chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components without breaking chemical bonds. Chemical substances can be solids, liquids, gases, or plasma
What is happening when scientists use their five senses to learn new
information?
A. Drawing a conclusion
B. Forming a hypothesis
C. Making observations
C
D. Making predictions
VIOUS
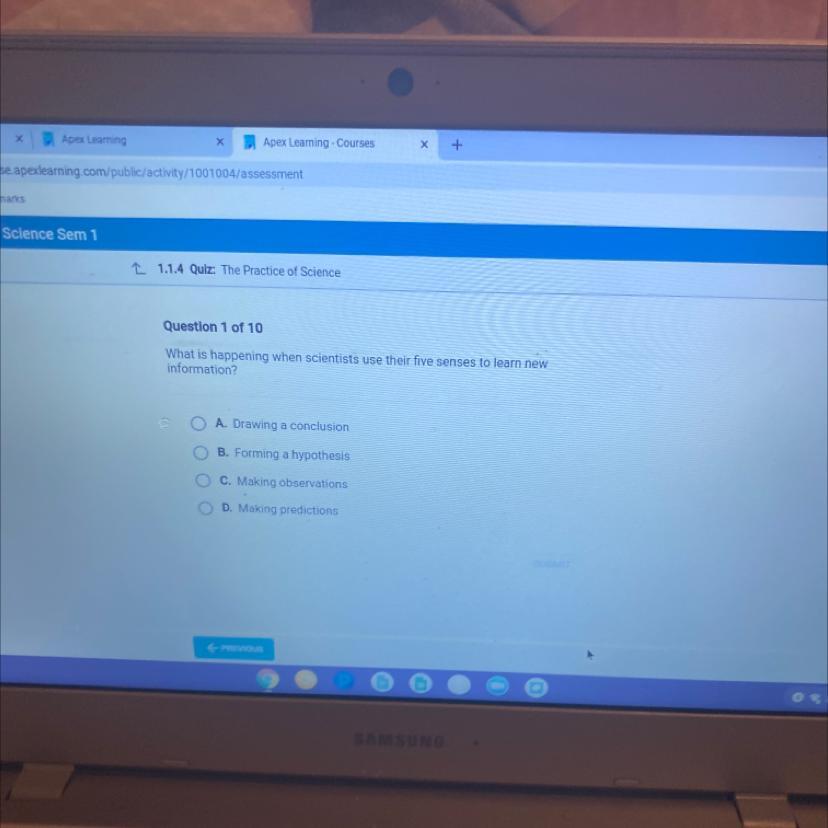
Answers
Answer:
A, drawing a conclusion
Explanation:
Potassium sulfate has a molecular mass of 174.3 g/mol. How many grams of potassium sulfate do you need to dissolve in 0.987 liters of water to prepare a solution with 0.735 molarity?
Answers
The mass of the nitrate is 126.4 g
What is the molarity?Molarity is a measure of the concentration of a solution, defined as the number of moles of solute dissolved in one liter of solution. It is usually represented by the symbol "M" and has units of moles per liter (mol/L).
We know that;
Number of moles = Mass/Molar mass
Number of moles = Concentration * volume
Mass/Molar mass = Concentration * volume
m/174.3 = 0.735 * 0.987
m = 0.735 * 0.987 * 174.3 =
m = 126.4 g
Molarity is an important concept in chemistry, as it allows chemists to precisely measure the concentration of a solution and to make accurate calculations in various chemical reactions.
Learn more about concentration:https://brainly.com/question/10725862
#SPJ1
How much energy would it take to heat a section of the copper tubing that weighs about 535.0 g , from 15.29 ∘C to 21.30 ∘C ? Copper has a specific heat of 0.3850 (J/g)⋅∘C.
Answers
Answer:
1238 J
Explanation:
Step 1: Given data
Mass of copper (m): 535.0 gSpecific heat of copper (c): 0.3850 J/g.°CInitial temperature: 15.29°CFinal temperature: 21.30°CStep 2: Calculate the change in the temperature
We will use the following expression.
ΔT = 21.30°C - 15.29°C = 6.01°C
Step 3: Calculate the energy required to heat the temperature of the copper tubing (Q)
We will use the following expression.
Q = c × m × ΔT
Q = 0.3850 J/g.°C × 535.0 g × 6.01°C
Q = 1238 J
What happens to the electric current in a series circuit when voltage decreases
Answers
Convert 3564 to Scientific Notation:
*
Answers
Answer:
3564 = 3.564 x 10^3
Explanation:
i.e expresing the value in standard form.
Enter a balanced equation for the reaction of KOH and Cu(NO3)2 . Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no precipitate is formed.
Answers
For each compound, the states (aq) for aqueous, or dissolved in water, and (s) for solid, or precipitate created, are used to denote their states.
What occurs when sodium hydroxide interacts with copper II nitrate?A mixture made up of a sodium nitrate solution and a copper(II) hydroxide precipitate is created when copper(II) nitrate and sodium hydroxide solutions are combined.
KOH and Cu(NO3)2 react, and the balanced equation for the reaction is
2KOH(aq) + Cu(NO3)2(aq) Cu(OH)2(s) + 2KNO3 (aq)
Copper(II) hydroxide (Cu(OH)2) and potassium nitrate are produced in this reaction between potassium hydroxide (KOH) and copper(II) nitrate (Cu(NO3)2) (KNO3).
To know more about aqueous visit:-
https://brainly.com/question/30215562
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
using the ideal gas law, PV = nRT, in which R is 8.31 (L•kPa/mol-K), what would the temperature be if 0.75 moles of helium gas in a 2.0 L container have a pressure of 202.65 kPa
Answers
Answer:
65.0 K
Explanation:
To find the temperature, you need to use the Ideal Gas Law:
PV = nRT
In this equation,
-----> P = pressure (pKa)
-----> V = volume (L)
-----> n = moles
-----> R = Ideal Gas Constant (8.31 L*kPa/mol*K)
-----> T = temperature (K)
You can plug the given values into the equation and simplify to find the temperature.
P = 202.65 pKa R = 8.31 L*kPa/mol*K
V = 2.0 L T = ? K
n = 0.75 moles
PV = nRT
(202.65 pKa)(2.0 L) = (0.75 moles)(8.31 L*kPa/mol*K)T
405.3 = (6.2325)T
65.0 K = T
A student combined 45 mL of 500 M KMnO₄ solution with 16.10 mL of a 010 M KMnO₄ solution. Calculate the concentration of the final solution.
Answers
The concentration of the final solution is 368.26 M.
To calculate the concentration of the final solution, we need to use the following formula:
C₁V₁ + C₂V₂ = C₃V₃
where:
C₁ = concentration of the first solution (500 M)
V₁ = volume of the first solution (45 mL)
C₂ = concentration of the second solution (0.10 M)
V₂ = volume of the second solution (16.10 mL)
C₃ = concentration of the final solution (unknown)
V₃ = total volume of the final solution (45 mL + 16.10 mL = 61.10 mL)
Substituting the values given, we get:
(500 M)(45 mL) + (0.10 M)(16.10 mL) = C₃(61.10 mL)
22500 + 1.61 = 61.10C₃
22501.61 = 61.10C₃
C₃ = 22501.61 ÷ 61.10
C₃ = 368.26 M
learn more about concentration here:
https://brainly.com/question/30639206
#SPJ1
How many moles of oxygen are present in a cylinder of 25.0 liters at a temperature of 0. °C and a pressure of 1.00 atm
Answers
Answer:
1.12 moles
Explanation:
To find the amount of moles, you need to use the Ideal Gas Law:
PV = nRT
In this equation,
-----> P = pressure (atm)
-----> V = volume (L)
-----> n = moles
-----> R = Ideal Gas constant (0.08206 atm*L/mol*K)
-----> T = temperature (K)
After converting the temperature from Celsius to Kelvin, you can plug the given values into the equation and solve for "n".
P = 1.00 atm R = 0.08206 atm*L/mol*K
V = 25.0 L T = 0. °C + 273 = 273 K
n = ? moles
PV = nRT
(1.00 atm)(25.0 L) = n(0.08206 atm*L/mol*K)(273 K)
25.0 = n(22.4)
1.12 = n
Question 2(Multiple Choice Worth 2 points)
(01.02 MC)
A team of researchers is working on a project to make a new kind of carbonless fuel. During their experiment, there is an explosion that destroys the lab. While they
are cleaning the debris, they discover three pieces of frozen metal. The researchers were excited to report that they had discovered a fuel that burns so clean it
becomes cold. Which statement best explains why this scenario represents "bad" science?
O The frozen metal was discovered through biased research practices.
O The clean fuel experiment lacks controlled variables due to the explosion.
O
The experiment is replicable because the testing conditions are included.
O
The result of clean fuel production, the frozen metal, is not observable.
Answers
They shouldn't have made a choice based on the frozen metals because scientific research is based on accepted procedures, not on personal bias.
Briefing:
A researcher that is biased is unable to look at experimental observations objectively before drawing any conclusions. Bias is the biggest trap that needs to be avoided when performing a scientific investigation.
When there is a discrepancy between the observation and interpretation of data in a scientific investigation, a bias is introduced.
In the instance of this inquiry, the researchers were already interested in determining whether it was possible to obtain a fuel without carbon. They reached a lobe-sided, unscientific conclusion because they forgot to fully study the chunks of frozen metal since their brains were so clouded by this bias.
To know more about metals, visit:
https://brainly.com/question/29404080
#SPJ1
To test for a ammonia, which of these should be held over the test tube Group of answer choices dry red litmus paper dry blue litmus paper moist red litmus paper moist blue litmus paper
Answers
In order to test for ammonia, a moist red litmus paper should be held over the test tube containing the ammonia. Option 3.
Testing for ammoniaAmmonia is a substance that is alkaline in nature. All alkaline substances are able to turn red litmus paper to blue. This is in opposition to acidic substances that turn blue litmus paper to red.
Thus, the first test that can be used to determine if a substance is ammonia would be to hold a moist red litmus paper over the test tube containing the suspected substance.
Since ammonia is gas at room temperature, the substance suspected to be ammonia will diffuse from the test tube to turn the moist red litmus paper to blue.
Other methods to test for ammonia include passing a test tube containing hydrochloric acid over the test tube containing the substance. A white fume of ammonium chloride will confirm the presence of ammonia.
More on tests for ammonia can be found here: https://brainly.com/question/29345206
#SPJ1
Describe the differences between lead (II) chloride and lead (IV) chloride in terms of the charges on the lead and chloride ions in each compound. Then, write the chemical formulas for each compound.
Answers
In lead (II) chloride, also known as plumbous chloride, the lead ion has a charge of +2 and the chloride ion has a charge of -1. The chemical formula for lead (II) chloride is PbCl2.
In lead (IV) chloride, also known as plumbic chloride, the lead ion has a charge of +4 and the chloride ion has a charge of -1. The chemical formula for lead (IV) chloride is PbCl4.
So the key difference between these two compounds is the oxidation state of the lead ion. In lead (II) chloride, the lead ion has a +2 oxidation state, while in lead (IV) chloride, the lead ion has a +4 oxidation state.
Answer:
Explanation:
Lead (II) chloride and lead (IV) chloride have different charges on the lead and chloride ions in each compound.
In lead (II) chloride, also known as plumbous chloride, the lead ion has a charge of +2 and the chloride ion has a charge of -1. The chemical formula for lead (II) chloride is PbCl2.
In lead (IV) chloride, also known as plumbic chloride, the lead ion has a charge of +4 and the chloride ion has a charge of -1. The chemical formula for lead (IV) chloride is PbCl4.
So the key difference between these two compounds is the oxidation state of the lead ion. In lead (II) chloride, the lead ion has a +2 oxidation state, while in lead (IV) chloride, the lead ion has a +4 oxidation state.
Given 0.38 grams of N2, how many grams of NaN3 are needed
Answers
What consists of water molecules that have escaped or evaporated from the body of water
Answers
Water molecules that have escaped or evaporated from the body of water then water get excited and they begin to gain kinetic energy
A water molecule has a three atom two hydrogen and one oxygen atom and that's why water is sometime refred to as H₂O and a single drop of water contain billion drop of molecule and evaporation is the process in which given to the change that occurs to a substance that changes it from its liquid state to a gaseous state
When water get evaporates the molecule of water in a glass will get excited and they begin to gain kinetic energy and vigorously hit the wall of glass
Know more about evaporation
https://brainly.com/question/7481738
#SPJ1
How does publishing in scientific journals help ensure that science leads to
reliable results?
A. It allows results to be reviewed and reproduced by other
scientists.
B. It allows the public to decide if science is worthwhile.
C. It shows which experiments are the most popular with scientists.
D. It gives scientists a reason to do more experiments.
Answers
Answer:
A. it allows results to be reviewed and reproduced by other scientists.
In which of the following compounds would the carbon-carbon bond angle diverge the greatest from the ideal tetrahedral angle?
Answers
Cyclopropane of the following compounds would the carbon-carbon bond angle diverge the greatest from the ideal tetrahedral angle.
What is Cyclopropane?The cycloalkane or cyclopropane has the chemical formula (CH2)3, and cyclopropane is made up of three methylene groups that are joined together to create a ring. The structure experiences significant ring strain as a result of the ring's modest size.
Use of cyclopropane:Since 1934, colorless gas has been used as a general anesthetic in medicine. Cyclopropane does not impair breathing and does not irritate mucosal membranes. Cyclopropane anesthesia is typically quickly induced and smoothly exhaled.To know more about Cyclopropane visit
https://brainly.com/question/18521496
#SPJ4
The complete question is:
In which of the following compounds would the carbon-carbon bond angle diverge the greatest from the ideal tetrahedral angle?
a. cyclodecane
b. cyclooctane
c. cyclopentane
d. cyclopropane
Please help me ASAP

Answers
which of the materials above are mixtures
Answers
Answer:
Even though i cannot see the image, In chemistry, a mixture is a material made up of two or more different chemical substance/substances which are not chemically combined. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions and colloids.
Explanation:
The energy required to dissociate the Cl2 molecule to Cl atoms is 239 kJ/mol Cl2. If the dissociation of a Cl2 molecule were accomplished by the absorption of a single photon whose energy was exactly the quantity required, what would be its wavelength (in meters)?
Answers
Explanation:
The energy of a single photon can be calculated using the equation E = hc/λ, where E is the energy of the photon, h is Planck's constant (6.626 x 10^-34 J.s), c is the speed of light (2.998 x 10^8 m/s), and λ is the wavelength of the photon.
To dissociate one mole of Cl2 molecules, we need 239 kJ of energy. This corresponds to 239,000 J/mol of Cl2.
Now we can use the relationship between energy and the number of photons absorbed: E = Nhf, where N is the number of photons absorbed, h is Planck's constant, and f is the frequency of the absorbed photons.
We can relate the frequency of the absorbed photon to its wavelength using c = λf. Solving for f gives f = c/λ.
Combining these equations, we get E = Nh(c/λ), or N = E/(hc/λ). Substituting the value of E for the energy required to dissociate one mole of Cl2, we get:
N = (239,000 J/mol) / [(6.626 x 10^-34 J.s) x (2.998 x 10^8 m/s) / λ]
Solving for λ, we get:
λ = hc / (239,000 J/mol) = (6.626 x 10^-34 J.s x 2.998 x 10^8 m/s) / 239,000 J/mol
λ = 8.44 x 10^-7 m
Therefore, the wavelength of the photon required to dissociate one Cl2 molecule is approximately 8.44 x 10^-7 meters (or 844 nanometers).
Why water can be unsafe in a pool?
Answers
Answer:People can drown or call or slip in
Explanation:
