which of the following describes a physical property of copper metal? answer unselected at high temperatures, copper forms a black copper oxide. unselected copper metal is ductile. it can be ulled into thin wires. unselected copper slowly oxidizes to form a green patina, like that on the statue of liberty. unselected copper does not react with water.
Answers
physical property of copper metal describes by the copper metal is ductile. it can be pulled into thin wires.
A physical property is a characteristic that can be observed or measured without changing the composition. Density is another illustration of a physical characteristic. Your coin is still made of the same material as it was before it was dropped into the fountain, despite the fact that it may be a little damp. Additional examples of physical characteristics include color, mass, smell, boiling point, volume, and temperature. Some physical characteristics, like density and color, can be observed without changing the physical state of the material being studied. Only when matter undergoes a physical transformation can other physical properties, like the melting point of iron or the freezing point of water, be observed.
To know more about Physical properties visit : https://brainly.com/question/2116116
#SPJ4
Related Questions
Intermolecular forces exist between what?
Answers
Answer:
Intramolecular forces are the forces that hold atoms together within a molecule. Intermolecular forces are forces that exist between molecules.
Explanation:
WILL GIVE 10 PTS AND BRAINIEST FOR THE RIGHT ANSWER PLEASE HELP!!!
____ is important for making amino acids, proteins, and the chlorophyll a plant uses to carry out photosynthesis.
A. Boron
B. Nitrogen
C. Potassium
D. Phosphorus
E. Calcium
Answers
Answer:
c
Explanation:
The graph shows five data points collected in an investigation of the relationship between the concentration of alcohol dissolved in water and its density. The relationship was expected to be linear. Which of the data points most likely resulted from an error in procedure? a 1 b 2 c 4 d 5
Answers
In comparison to modern, highly accurate density meters or pycnometers, hydrometers are far less accurate and temperature.
Thus, Although they require very large sample sizes, hydrometers are rather simple to operate. Usually, 300 to 500 ml per measurement are required. Hydrometers frequently require calibration off-site as well.
With measurements taken by eye, user error is a major issue, and temperature management is especially challenging. Inaccurately bringing and maintaining samples at temperature might take a long time, and once more, user perception of temperature levels is used to determine temperature levels.
Pycnometers and hydrometers have a further problem in that the findings of alcohol measurement are challenging to evaluate and record.
Thus, In comparison to modern, highly accurate density meters or pycnometers, hydrometers are far less accurate and temperature.
Learn more about Temperature, refer to the link:
https://brainly.com/question/7510619
#SPJ1
What are the possible values of 1 and m for
n=4 ?
Answers
Answer:
If n = 4, then the possible values of 1 and m depend on the equation or expression being used. Without more information, it is impossible to determine what the possible values of 1 and m might be. Can you please provide more context or information about the problem you are trying to solve?
I need help asap please help me
Answers
Two hydrogen atoms form a hydrogen molecule by which?Loosing electrons Sharing protons Gaining electrons Sharing electrons
Answers
Step 1 - Deciding between ionic and covalent bond
In the ionic bond, atoms gain or lose electrons, forming ions, which attract themselves thus resulting in a very strong chemical bond. In the covalent bond, on the other hand, electrons are shared by two atoms, resulting in a molecule.
The ionic bond usually happens between a metal (low ionization energies) and a non-metal (high electron affinities), while covalent bond happens between two or more non-metal atoms.
Therefore, looking at the atoms in the substance we can discover whether it is an ionic or covalent substance.
Step 2 - Using this information to answer the exercise
Note that hydrogen gas (H2) is a molecule. Therefore, its bonds must be covalent. That is so because H is a non-metal. Whenever two non-metals bond, be they different or the same, a covalent bond arises.
Since a covalent bond is the sharing of electrons between atoms, the correct answer is item d) sharing electrons.
100 Points} Name the following compounds from the structures given (images shown below)
1.
2.
3.
4.
5.
6.
Unfortunately, they're not multiple choice, so I have no possible answers to list, I believe 1. might be "2-methylhexane" but I'm unsure how to write the double bond that's shown in the structure, thanks! :)
Edit; the screenshots posted out of order, my apologies :(


Answers
Answer:
1.) There are 6 carbons in the longest possible parent chain (hex-). Since there is a double bond, this is an alkene. The lowest possible carbon the double bond consists of is the 2nd carbon. There is also a methyl group on the 2nd carbon. All together, this makes the structure: 2-methyl-2-hexene.
2.) There are 9 carbons in the longest possible parent chain (non-). The lowest possible carbons the methyl groups are on are the 3rd and 5th carbons. The lowest possible carbon the ethyl group is located on is the 4th carbon. Remember, branches are listed alphabetically. All together, this makes the structure: 4-ethyl-3,5-dimethylnonane.
3.) There are 7 carbons in the longest possible parent chain (hept-). There is a triple bond, making this an alkyne. The lowest possible carbon the triple bond consists of is the 2nd carbon. The lowest possible carbon the methyl group is on is the 4th carbon. All together, this makes the structure: 4-methyl-2-heptyne.
4.) There are 10 carbons in the longest possible parent chain (dec-). The lowest possible carbon the propyl group is on is the 5th carbon. All together, this structure is: 5-propyldecane.
5.) There are 4 carbons in the longest possible parent chain (but-). The lowest possible carbon the methyl group is on is the 2nd carbon. All together, this makes the structure: 2-methylbutane.
6.) There are 5 carbons in the longest possible parent chain (pent-). There is a double bond, making the molecule an alkene. The lowest possible carbon the double bond consists of is the 2nd carbon. The lowest possible carbon the methyl group is on is the 2nd carbon. All together, this makes the structure: 2-methyl-2-pentene.
PLEASE HELP I WILL MARK BRAINLIEST!!!
Reactions In Our World Lab Report
Answers
Reactions in our natural world range from classical chemical reactions to nuclear reactions.
What is a chemical reaction?A classical chemical reaction is a natural process that occur when one or more elements called reactants change to form one or more products.
Chemical reactions can be speed up by the action of enzymes, which are biological catalysts capable of lowering the activation energy of the reactions.
On the other hand, nuclear reactions occur when atoms react among them to generate new elements.
Nuclear reactions can be classified into fusion reactions where atomic nuclei fuse and fusion reactions where an atomic nucleus gives rise to two different atoms.
In conclusion, Reactions in our natural world range from classical chemical reactions to nuclear reactions.
Learn more about chemical reactions here:
https://brainly.com/question/11231920
#SPJ1
Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chloride and liquid water . Suppose 14. g of hydrochloric acid is mixed with 6.55 g of sodium hydroxide. Calculate the minimum mass of hydrochloric acid that could be left over by the chemical reaction. Round your answer to significant digits.
Answers
Answer:
8.02 g of HCl could be left over by the chemical reaction
Explanation:
We propose the reaction:
HCl(aq) + NaOH (s) → NaCl (aq) + H₂O (l)
Ratio is 1:1. First of all, we determine the moles of reactants:
14 g . 1mol / 36.45g = 0.384 mol of acid
6.55 g. 1mol / 40g = 0.164 mol of base
If a determined mass of HCl, could be left; this means that the acid is the excess reagent.
For 0.164 moles of NaOH, we need 0.164 moles of HCl.
As we have 0.384 moles, (0.384 - 0.164) = 0.220 moles of acid are left over in the reaction. We convert the moles to mass:
0.220 mol . 36.45 g /1mol = 8.02 g
help me please ..............

Answers
Answer:
C
Explanation:
Answer:
Hey, you have to solve it by Stichiometry.....answer is option A.... I have explained every option for your understanding.
Explanation:
(a.)**For option A, the volume of CO2 produced at STP will be 5.6 not 30.8 by solving equation for part I
By Stichiometry,
moles of CO2, n=11.2/22.4 = 0.5
Volume of CO2, V=11.2×0.5 =5.6
(b.)**For option B, the statement is correct
n(moles) of O2 =32/32 = 1 mol
we have to use stichiometry to find moles of CO2
n= 1/2 × 1 (coefficient of CO2/coefficient of O2) × mol of O2 (coz O2 is limiting reagent in 2nd option)
n= 0.5 mol
Mass= n × Molecular Mass
Mass= 0.5 × 44
Mass= 22g
(c.)**option C, is correct acc. to statement
(d.)**option D, is correct acc. to statement
(e.)**option E, is correct acc. to statement
Answer to this question is A part.
Hope it helps......
Please help me I. Eg you

Answers
Answer:
fehrf beviubeibchvbiscv kie v sjb rlj jsrubw
Explanation:
A student conducted an experiment 4 times. His results were very close to each other each time he ran the experiment and
were very close to the true or actual value. His results showed
A. None of these answers are correct
B. poor accuracy and poor precision
C. good accuracy and good precision
D. poor accuracy and good precision
E. good accuracy and poor precision
Answers
Answer:
d is the answer have a good one
Why is there an imbalance in the carbon cycle?
Answers
What is a reaction that alkanes undergo ?
A. precipitation reactions
B. combustion reaction
C. acid base reactions
D. all of the above
Answers
All combustion reactions have oxygen as a reactant. (2 points)
Group of answer choices
True
False
Answers
Answer:
true
Explanation:
They all have a hydrocarbon plus oxygen.
The given statement that "all combustion reactions have oxygen as a reactant" is true. Combustion is a type of chemical reaction where a fuel (usually a hydrocarbon) combines with oxygen gas to produce heat, light, and new chemical compounds, such as carbon dioxide and water.
For combustion to occur, there must be a fuel source, oxygen, and a source of ignition, such as a spark or heat. Oxygen acts as a reactant because it combines with the fuel source to produce the new compounds. Without oxygen, the reaction cannot occur. It is important to note that not all reactions involving oxygen are combustion reactions. For example, rusting of iron is a reaction that involves oxygen, but it is not a combustion reaction.
In combustion reactions, the heat and light produced are often used for industrial processes, transportation, or heating. However, the reaction can also be destructive if not controlled, such as in wildfires or explosions. In conclusion, all combustion reactions have oxygen as a reactant, as it is necessary for the reaction to occur and produce the desired products.
For more such questions on combustion
https://brainly.com/question/13251946
#SPJ11
Element Q is a theoretical nonmetal with atomic number 59. Consider the isotope: Q-123. How many neutrons are in an atom of Q-123 if the atom has a charge of -1?
Answers
There are 64 neutrons in an atom of Q-123 with a charge of -1.
Since the isotope Q-123 has an atomic number of 59, we know that it has 59 protons. The charge of -1 tells us that the atom has one more electron than protons, so it has 60 electrons.
To find the number of neutrons, we need to subtract the atomic number (59) from the mass number (123):
Number of neutrons = Mass number - Atomic number
Number of neutrons = 123 - 59
Number of neutrons = 64
Hence there are 64 neutrons in an atom of Q-123 with a charge of -1. It is important to note that the charge does not affect the number of neutrons in the atom, only the number of electrons. The number of protons and neutrons in the nucleus of an atom determine its identity and chemical properties.
for more questions on atom
https://brainly.com/question/26952570
#SPJ11
Calculate the partial
pressure of argon in a
gas mixture with a total
pressure of 2.4 atm.
The partial pressures
of the other gases are:
O2 = 128.0 mmHg
He = 167.5 mmHg and
Ne= 760.0 mmHg
Answers
The required partial pressure of argon gas present in the mixture is 1.02 atm.
What is Dalton law of gas?Dalton's law of gas states that total pressure of any mixture of gas is sum of the partial pressure of all the gases present in that mixture.
Given that,
Total pressure of mixture = 2.4 atm
Partial pressure of argon = ?
Partial pressure of oxygen = 128 mmHg = 0.168 atm
Partial pressure of helium = 167.5 mmHg = 0.220 atm
Partial pressure of neon = 760 mmHg = 1 atm
On putting all these values according to the definition we get the partial pressure of argon gas as:
Partial pressure of argon = 2.4 - (0.168 + 0.22 + 1) = 1.02 atm
Hence required partial pressure of argon gas is 1.02 atm
To know more about Dalton's law, visit the below link:
https://brainly.com/question/8040766
#SPJ1
11. In a reaction from number 10, 65.0g of Ni(NO3)2 is reacted with 58.0g KOH. Which is
the limiting reactant? Show your work for credit. (4pts)
Answers
Answer:
Ni(NO3)2 is the limiting reactant.
Explanation:
- First, we balance the equation...
Ni(NO3)2 + 2 KOH ---> 2 KNO3 + Ni(OH)2
- Second, we find the moles of each substance...
65g Ni(NO3)2 / 182.703g Ni(NO3)2 = 0.356 mol Ni(NO3)2
58g KOH / 56.1056g KOH = 1.034 mol KOH
- Third, to make the molar ratio equal to each other for comparison, we either multiply KOH by 1/2 or multiply Ni(NO3)2 by 2 to compare the number of moles; because the Ni(NO3)2 to KOH molar ratio is 1 to 2. Note that the multiplication of moles is only for comparison. We do not use these multiplied values. We use the values from step 2...
0.356 mol Ni(NO3)2 * 2 = 0.712 mol Ni(NO3)2
0.712 mol Ni(NO3)2 < 1.034 mol KOH ... Ni(NO3)2 is the limiting reactant.
when a _____ is formed, the substances are chemically bonded and lose their original properties. they can be separated by physical means
Answers
When a compound is formed, the substances are chemically bonded and lose their original properties.
What is a compound?A compound is made up of two or more substances that are joined with chemical bonds. The compound that is formed is different from the substances. For example, water is a compound, made up of oxygen and hydrogen.
The compounds are of different types. They are covalent compounds, ionic compounds, and intermetallic compounds.
Thus, the substances that are chemically bonded and lose their original properties are called compounds.
To learn more about the compound, refer to the below link:
https://brainly.com/question/14658388
#SPJ1
What mass of water was produced if 350.0 L of carbon dioxide were made at STP? C3H8 (g) + 5O2(g) → 2CO2 (g) + 4H2O (g)
Answers
700 L of water was produced if 350.0 L of carbon dioxide were made at STP.
The quantitative relationship (ratio) between reactants and products in a chemical reaction that produces gases is known as gas stoichiometry. When the created gases are presumed to be ideal and their temperature, pressure, and volume are all known, gas stoichiometry is applicable.
The ideal gas equation is PV=nRT, where n is the number of moles and R is the gas constant, P is the pressure measured in atmospheres (atm), V is the volume measured in liters (L), and
Calculations based on stoichiometry assist scientists and engineers who work in the business world in estimating the number of items they will make using a particular process. They can also assist in determining if a product will be economical to produce.
Reduced growth, reproduction, and survivability for the consumer are typically the results of a significant stoichiometric imbalance between the primary producer and consumer.
To know more about stoichiometry refer to: https://brainly.com/question/9743981
#SPJ1
In nature, oxygen has three common isotopes. The atomic masses and relative abundances of these isotopes are given in the table below. Isotope Atomic Mass (amu) Relative Abundance O-16 15.995 99.759% O-17 16.995 0.037% O-18 17.999 0.204% Calculate the average atomic mass of oxygen. Show all of your calculations below.
Answers
Answer: The average atomic mass of oxygen is 15.999 amu
Explanation:
Mass of isotope O-16 = 15.995 amu
% abundance of isotope O-16= 99.759 % = \(\frac{99.759}{100}=0.99759\)
Mass of isotope O-17 = 16.995 amu
% abundance of isotope O-17 = 0.037% = \(\frac{0.037}{100}=0.00037\)
Mass of isotope O-18 = 17.999 amu
% abundance of isotope O-18 = 0.204% = \(\frac{0.204}{100}=0.00204\)
Formula used for average atomic mass of an element :
\(\text{ Average atomic mass of an element}=\sum(\text{atomic mass of an isotopes}\times {{\text { fractional abundance}})\)
\(A=\sum[(15.995\times 0.99759)+(16.995\times 0.00037)+(17.999 \times 0.00204)]\)
\(A=15.999\)
Thus the average atomic mass of oxygen is 15.999 amu
Answer:
Converting the percent abundance into decimal form, we get:
O-16: 99.759% = 99.759/100 = 0.9975
O-17: 0.037% = 0.037/100 = 0.00037
O-18: 0.204% = 0.204/100 = 0.0020
Average atomic mass of oxygen is:
(15.995) x (0.9975) + (16.995) x (0.00037) + (17.999) x (0.0020)
= 15.955 + 0.0062 + 0.0359
= 15.997 amu
Explanation:
From PLATO
Which of the following is NOT an accurate way to measure wavelength?
A. crest to trough
B. trough to trough
C. half crest to half crest
D. crest to crest
Answers
Answer: D
Explanation: crest to crest to the crust to crust to the west to west.
Please help me with this question please just help me with what is this is or synthesis singlereplacement
combustion
decomposition
double replacement

Answers
Answer:
1) Combustion
2)Synthesis
3)Single Replacement
4)Decomposition
5)Double Replacement
6)Synthesis
7)Double Replacement
8)Single Replacement
9)Combustion
10)Decomposition
Explanation:
Remeber, decomposisition is breaking things apart, synthesis is building them, cobusion will always make water and CO2 and will always burn O2, single replacemnt will have 1 thing replaced (single) and double replacement will have 2 things replaced (double).
Hope this helps!
Please help me with this
If somebody posts b.u.l.l.s.h.i.t. answers, please report them!!
Answers
Answer:
where is the question
Explanation:
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
ASAP PLEASE!!!B. Complete the drawing for the sample reaction below to show the law of conservation of
mass, when XY is produced.
+
->
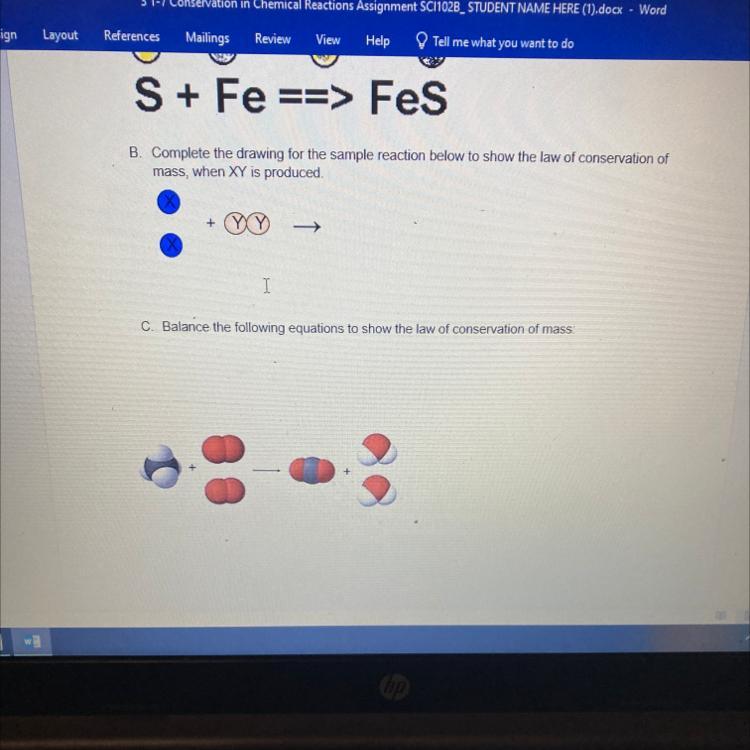
Answers
The complete reaction, according to the law of conservation of mass is:
XX + YY → 2XY
The Law of Conservation is a fundamental principle in chemistry and physics. It states that in a closed system, mass cannot be created or destroyed during a chemical reaction or a physical change. The total mass of the substances involved before the reaction or change must equal the total mass of the substances after the reaction or change.
This principle is based on the understanding that atoms are not created or destroyed, but they can combine or separate to form different substances.
Learn more about the law of mass conservation, here:
https://brainly.com/question/28711001
#SPJ1
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
What is a real life example of Charles’ law?
Answers
Hydrochloric acid and oxygen gas form water and chlorine gas. How many moles of oxygen are required to produce 4.59 moles of chlorine gas.
Answers
Answer:
2.295 moles of O2 are needed.
Explanation:
1st) It is necessary to write and balance the chemical reaction:
\(4HCl+O_2\rightarrow2H_2O+2Cl_2\)2nd) From the balanced reaction we can see that to produce 2 moles of chlorine gas (Cl2), 1 mole of oxygen gas (O2) is needed. So, with a mathematical rule of three we can calculate the amount of oxygen gas moles needed to produce 4.59 moles of Cl2:
\(\begin{gathered} 2\text{ moles Cl}_2-1\text{ mole O}_2 \\ 4.59\text{ moles Cl}_2-x=\frac{4.59\text{ moles Cl}_2*1\text{ mole O}_2}{2\text{ moles Cl}_2} \\ x=2.295\text{ moles O}_2 \end{gathered}\)Finally, 2.295 moles of O2 are needed
A solution of dispersant is made by taking 15.0 mL of a 50.0 mg/mL solution of Randyne and mixing it with 50.0 mL of water. Calculate the final concentration of the Randyne in this solution, in units of grams per milliliter.
Answers
Answer:
The final concentration of the Randyne in grams per milliliter = 0.011 g/mL
Explanation:
As we know
C1V1 = C2V2
C1 and C2 = concentration of solution 1 and 2 respectively
V1 and V2 = Volume of solution 1 and 2 respectively
Substituting the given values, we get -
\(50 * 15 = X * (15+50)\\X = 11.54\) mg/mL
The final concentration of the Randyne in grams per milliliter = 0.011 g/mL
The final concentration of the Randyne in this solution is 0.01 g /mL.
How to calculate dilutions?It is very important to know the dilution methods in a chemistry lab. The dilution from the stock solution can be prepared by using the formula,
\(C_1V_1 = C_2V_2\)
Where,
\(C_1\)- concentration of the stock solution
\(V_1\) - the volume of the stock solution
\(C_2\) - concentration of the diluted solution
\(V_2\) - the volume of diluted solution
Put the values in the formula,
\(50 \times 15 = C_2 \times 75 \\\\C_2 = \dfrac {750}{75 }\\\\C_2 = 10{\rm \ mg/mL \ \ \ or} \\\\ C_2 = 0.01 \rm \ g/mL\)
Therefore, the final concentration of the Randyne in this solution is 0.01 g /mL.
Learn more about dilution methods:
https://brainly.com/question/25307719