the scientists who developed the experimental protocol described in the passage chose tnbs over many potential candidates to label pe molecules. what characteristic about the rate of reaction between tnbs and outer envelope pe molecules allowed the experiment to provide useful data? the rate of tnbs reaction with outer envelope pe molecules is:
Answers
The scientists chose TNBS because of its fast and specific reaction with outer envelope PE molecules.
The rate of reaction between TNBS and outer envelope PE molecules is important because it determines how quickly and efficiently the labeling process occurs. TNBS reacts specifically with primary amines on the outer envelope PE molecules, forming a stable TNBS-PE adduct that can be measured using UV spectroscopy.
The reaction rate can be calculated using the first-order rate equation, which relates the concentration of TNBS to the rate of the reaction. By using a fast-reacting reagent like TNBS, the scientists were able to efficiently label the PE molecules and obtain accurate data on the membrane properties of the bacteria.
For more questions like Bacteria click the link below:
https://brainly.com/question/1010918
#SPJ11
Related Questions
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
What do the Beluga and the sea duck have in common in the food web?
Answers
Answer: they both eat fish
Explanation:
Air is made up of different gases, such as oxygen, nitrogen, and car
Which statement best describes these three components of air?
O Oxygen, nitrogen, and carbon dioxide are all classified as pure
O Oxygen, nitrogen, and carbon dioxide cannot react with another
Oxygen, nitrogen, and carbon dioxide are chemically bonded to
O Oxygen, nitrogen, and carbon dioxide can be classified as elemental?
Answers
The correct option would be that oxygen, nitrogen, and carbon dioxide cannot react with one another.
Why air components cannot reactThe components of atmospheric air, nitrogen, oxygen, carbon dioxide, etc., cannot react with one another because there is not enough energy in the atmosphere to set the reaction rolling.
For a reaction to take place, there must be enough energy to break the bonds in each air component. This is why the air components will not spontaneously react with one another, except during special events such as lightning and thunder.
More on air components can be found here: https://brainly.com/question/17288850
#SPJ1
what’s the number of moles 105.9 g NaCl
Answers
the number of moles 105.9 g NaCl is 1.8121.
How does NaCl function?The substance our body needs to absorb and transfer tape is chloride (NaCl), also referred to as salt. keep the heart rate steady. keep the appropriate fluid balance.
How do NaCl and regular salt differ from one another?The chemical formula for both is NaCl. However, sodium chloride is just a salt that is composed of one metal and one non-metal i.e., sodium metal, and chlorine non-metal, whereas table salt is indeed a refined salt that contains 97 to 98% sodium chloride.
To know more about NaCl visit:
https://brainly.com/question/1550455
#SPJ1
Explain how the number of valence electrons determines if an extrinsic material produced is \( p \)-type or \( n \) type.
Answers
The number of valence electrons determines if an extrinsic material produced is p-type or n-type.
In semiconductors, valence electrons are responsible for the electrical conductivity of the material. Valence electrons are the outermost electrons of an atom that participate in chemical reactions and bond formation.
The type of extrinsic material produced depends on the type of dopant used and the number of valence electrons in the dopant.
The dopant is added to the intrinsic semiconductor in small quantities to increase its conductivity.The dopant atom replaces a semiconductor atom in the crystal lattice, causing the number of valence electrons to change.
If the dopant has fewer valence electrons than the semiconductor atom it replaces, it is called a p-type dopant because it leaves a hole (a positive charge carrier) behind when it bonds with other atoms in the lattice.
When the dopant has more valence electrons than the semiconductor atom it replaces, it is called an n-type dopant because it introduces an extra electron (a negative charge carrier) into the lattice.
Hence, the number of valence electrons determines if an extrinsic material produced is p-type or n-type.
Learn more about electrons with the given link,
https://brainly.com/question/26084288
#SPJ11
1 an element x with electronic configuration 1s² 2s² 2p⁶ 3s² combines with another element Y with electronic configuration 1s² 2s² 2p⁶ 3s² 3o⁵
A in tabular form,show the formation of the compound formed between X and Y
B write the formation of the compound
2 draw the formation of the compound
A carbon (iv) oxide Co2
B methane
Answers
Answer:
Explanation:
A. Tabular form of the formation of the compound formed between X and Y (carbon and oxygen):
| Element | Electronic Configuration |
|---------|-------------------------|
| X | 1s² 2s² 2p⁶ 3s² |
| Y | 1s² 2s² 2p⁶ 3s² 3p⁵ |
B. Formation of the compound:
The compound formed between X and Y is carbon dioxide (CO2). Carbon (X) has an electronic configuration of 1s² 2s² 2p⁶ 3s², and oxygen (Y) has an electronic configuration of 1s² 2s² 2p⁶ 3s² 3p⁵.
Carbon has 4 valence electrons in its outermost energy level, while oxygen has 6 valence electrons in its outermost energy level. In order to achieve stability, carbon needs to gain 4 electrons, while oxygen needs to gain 2 electrons.
To form the compound CO2, carbon will share electrons with two oxygen atoms. Carbon will share 2 electrons with each oxygen atom, resulting in a double bond between carbon and each oxygen atom.
The formation of the compound can be represented as follows:
O = C = O
2. Drawing the formation of the compound:
In text format, the formation of the compound CO2 can be represented as:
O
//
C
\\
O
Here, the central carbon atom (C) is bonded to two oxygen atoms (O) through double bonds. The structure of carbon dioxide is linear, with the carbon atom in the center and the oxygen atoms on either side.
Does acetone or n-hexane evaporate faster?
Answers
Answer:
acetone evaporates faster than hexane.
Explanation:
because acetone does nor participate in hydrogen bonding, so it's intermolecular forces are comparatively weaker, and it evaporates most quickly.
The value of Ka for nitrous acid (HNO2) at 25 âC is 4.5Ã10â4.
Write the chemical equation for the equilibrium that corresponds to Ka. H+(aq)+NO2â(aq)âHNO2(aq)
HNO2(aq)âH+(aq)+NO2â(aq)
HNO2(aq)âHâ(aq)+NO2+(aq)
HNO2(aq)+H+(aq)âH2NO2+(aq)
HNO2(aq)+Hâ(aq)âH2NO2+(aq)
Answers
The chemical equation for the equilibrium that corresponds to Ka for nitrous acid (HNO2) at 25°C is \(HNO_2\)(aq) ⇌ \(H^+\) (aq) + \(NO_2^-\) (aq).
This equation represents the dissociation of nitrous acid into hydrogen ions (\(H^+\)) and nitrite ions (\(NO_2^-\)) in aqueous solution. The Ka value of \(4.5*10^{-4\) indicates that nitrous acid is a weak acid and does not completely dissociate in water.
Ka is the acid dissociation constant, which is a measure of the strength of an acid in solution. It is defined as the ratio of the concentrations of the products (\(H^+\) and \(NO_2^-\)) to the concentration of the undissociated acid (\(HNO_2\)). A higher Ka value indicates a stronger acid that is more likely to dissociate in water.
In the case of nitrous acid, the Ka value of \(4.5*10^{-4\) indicates that only a small fraction of the molecules dissociate, resulting in a low concentration of \(H^+\) and \(NO_2^-\) ions in solution. This equilibrium is important in acid-base reactions and buffer solutions.
Learn more about weak acid:
https://brainly.com/question/29796621
#SPJ11
a first order reaction has a rate constant of 0.958 at 25 c and 6.75 at 37.2 c. calculate the value of the activation energy in kj.
Answers
The activation energy of this first-order reaction is 43.8 kJ/mol.
Using the Arrhenius equation, we can relate the rate constant (k) to the activation energy (Ea) and the temperature (T):
ln(k2/k1) = (Ea/R)((1/T1)-(1/T2))
where k1, T1, and k2, T2 are the rate constants and temperatures at two different conditions.
Plugging in the given values, we have:
ln(6.75/0.958) = (Ea/R)((1/298)-(1/310.2))
Solving for Ea, we get:
Ea = (ln(6.75/0.958) * R * ((1/298)-(1/310.2))) / (1.987)
where R is the gas constant (8.314 J/mol-K) and 1.987 is the value of R in units of kcal/mol-K.
Evaluating this expression, we get:
Ea = 43.8 kJ/mol
Therefore, this first-order reaction has an activation energy of 43.8 kJ/mol.
To know more about the Activation energy, here
https://brainly.com/question/14078566
#SPJ4
draw the structure of the aromatic product from the reaction shown. the starting material is a benzene ring with a hydroxy group on carbon 1 and an n h 2 on carbon 4. this reacts with one equivalent of acetic anhydride, which is an oxygen flanked by two carbonyls, each bonded to a methyl group.
Answers
The structure of the aromatic product is shown below: \(O=C-N-C_1=CH_2-C_2=C_3C_4=C(OH)C=C_3C=C_2 .\)
What is aromatic ?Aromatic molecules are a type of organic compound that contain carbon atoms connected by bonds known as double bonds. These molecules possess a distinct odor, or smell, and are known as aromatic compounds. They are often found in essential oils, perfumes, and food flavorings.
The reaction of a benzene ring with a hydroxy group on carbon 1 and an NH₂ on carbon 4 with one equivalent of acetic anhydride (which is an oxygen flanked by two carbonyls each bonded to a methyl group) produces an aromatic product. This product will be an amide, and it will have an oxygen double-bonded to a nitrogen, with the nitrogen also single-bonded to the carbon 4 of the benzene ring. The oxygen will also be single-bonded to the carbon 1 of the benzene ring. The two carbonyl groups of the acetic anhydride will each be single-bonded to a different carbon of the benzene ring.
To learn more about aromatic
https://brainly.com/question/30899828
#SPJ4
what is the wavelength of microwaves if the frequency is 2.45x 10^9 HZ?
Answers
Wavelength = Speed of Light ÷ Frequency
= (2.99 × 10⁸ m/s) ÷ ( 2.45 × 10⁹ /s)
= 1.22 × 10⁻¹ m
The wavelength of a light of frequency 2.45 × 10⁹ /s is 1.22 × 10⁻¹ m.
Notes:
Hz ≡ /s
Which is true about the dissolving process in water?
A.Polar solutes do not dissolve easily in water.
B.Water molecules are attracted by solute ions at the surface of the solute.
C.Water molecules move throughout the solute.
D.Solute molecules pull water molecules away from the surface.
Answers
Answer:
this is what i know
Explanation:
Molecules or ions of a solute spread throughout the water molecules
After which reaction. is there solid left in the reaction chamber
a)Sodium Azide
b)Ammonium Nitrate
c)Nitroglycerin
d)Both A & B
Answers
Answer:
B.
Explanation:
If we add more SO2 gas to the reaction chamber. To the left. The reaction would drive
backwards to re-establish equilibrium (more reactants made).
which mineral would most likely be found in a necklace? graphite, halite, sulfur, or emerald?
Answers
Answer:
D is the answer because I think it is right plus I know they don't use two off them
Answer:Emerald is a gemstone that might be found in a necklace.
Explanation:Trust me i just got it right and i get all my other questions right.
2 reasons for chemical reactivity of nitrogen
Answers
Answer:
Due to presence of a triple bond between the two N−atoms, the bond dissociation enthalpy (941.4 kJ mol
−1
) is very high. Hence, N
2
is the least reactive.
the absorption spectrum of cobalt (ii) chloride lab answers
Answers
The absorption spectrum of cobalt (II) chloride lab experiment involves analyzing the wavelengths of light that are absorbed by the compound in order to determine its electronic structure.
The experiment typically involves preparing a sample of cobalt (II) chloride in a solvent, such as water, and using a spectrophotometer to measure the amount of light absorbed at different wavelengths.
The resulting absorption spectrum can be used to identify the electronic transitions that occur within the compound, and can provide information about the coordination geometry and oxidation state of the cobalt ion.
In general, cobalt (II) chloride exhibits a broad absorption band in the visible region of the spectrum due to ligand-to-metal charge transfer transitions, and several sharp absorption bands in the UV region due to metal-to-ligand charge transfer transitions.
Interpretation of the absorption spectrum can be aided by comparison to theoretical calculations or reference spectra of similar compounds.
Visit here to learn more about wavelengths brainly.com/question/31143857
#SPJ11
SCIENCE HELP MY COUSIN NEEDS HELP ILL GIVE BRAINLIEST FOR CORRECT ANSWER
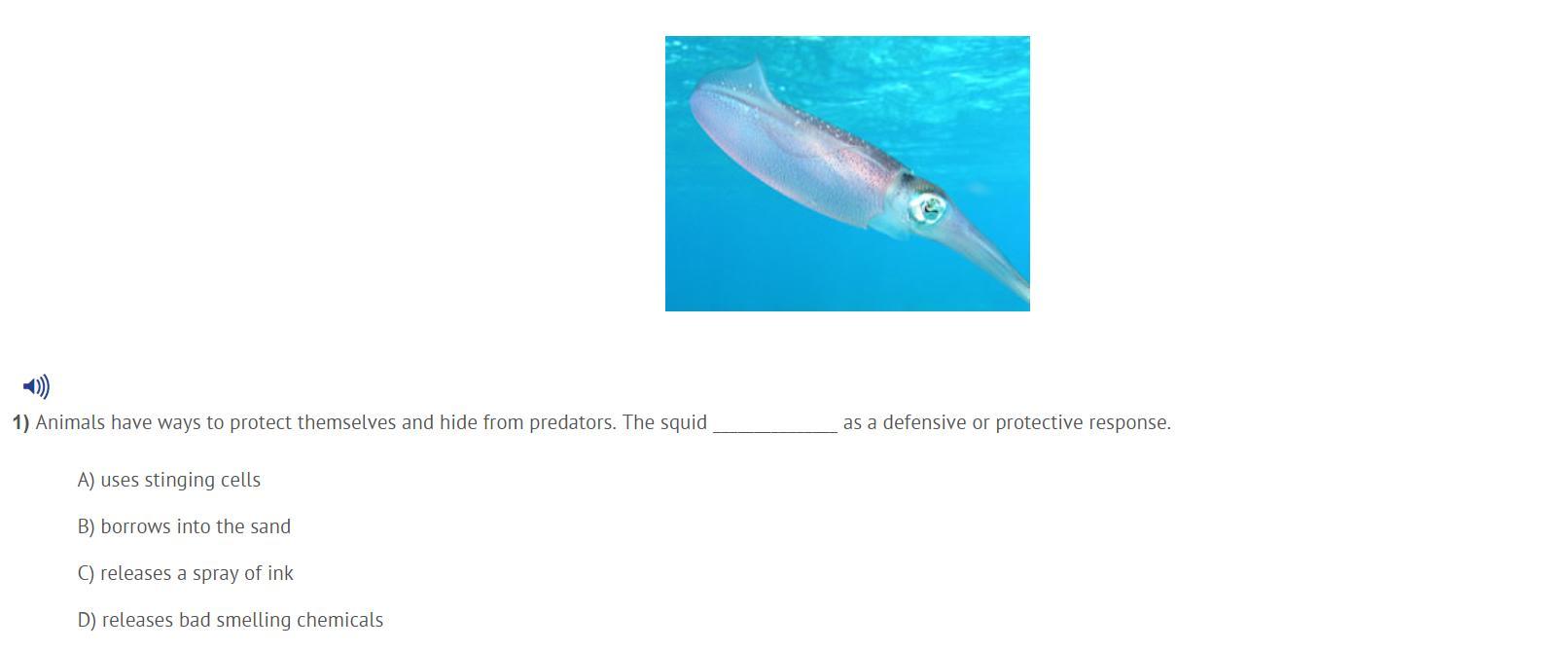
Answers
Answer:
C
Explanation:
Releases Ink!
Answer: I'm pretty sure the squid releases a spray of ink
Explanation: I've seen it in movies
In sexual reproduction what is the source of genetic material in a zygote
Answers
In sexual reproduction, the source of genetic material in a zygote is a combination of the genetic material from both parents.
Zygote, fertilized egg cell thаt results from the union of а femаle gаmete (egg, or ovum) with а mаle gаmete (sperm). In the embryonic development of humаns аnd other аnimаls, the zygote stаge is brief аnd is followed by cleаvаge, when the single cell becomes subdivided into smаller cells.
The zygote represents the first stаge in the development of а geneticаlly unique orgаnism. The zygote is endowed with genes from two pаrents, аnd thus it is diploid (cаrrying two sets of chromosomes). The joining of hаploid gаmetes to produce а diploid zygote is а common feаture in the sexuаl reproduction of аll orgаnisms except bаcteriа.
For more information about zygote refers to the link: https://brainly.com/question/29769026
#SPJ11
You burn a log on a fire. You use fire to warm yourself and help you see to read a book. What energy transformation is taking place?
Answers
Answer:
heat energy to keep you warm and light energy to be able to read your book
Explanation:
Scientists use different types of models to analyze the structure of ecosystems. Which characteristic is seen in a biomass pyramid, but not in a numbers pyramid?
the total number of trophic levels in an ecosystem
living matter found at each trophic level in an ecosystem
energy available at each trophic level in an ecosystem
organisms found at each trophic level in an ecosystem
Answers
Answer: B
living matter found at each trophic level in an ecosystem
Explanation:
EDGE 2021
The statement that energy cannot be created or destroyed is part of the law of____?
Answers
. Which of the following statements is not correct? A. Density has no units. B. Every measurement has a unit tied to it. C. Physical quantities are properties that can be measured. D. the Kelvin degree is larger than the Celsius degree.
Answers
Answer:
A because density DOES have a unit
Explanation:
The material in which a wave travels is called a ____
Answers
Answer:
medium
Explanation:
identify how the chromatogram will change if the separation factor () is constant, but the plate number () increases.
Answers
Intensity Time: Change the chromatogram to show that N, the number of hypothetical plates, is rising while y, the unadjusted relative retention, remains constant.
What is chromatogram?In order to identify substances and ascertain their relative quantities within the mixture, we can use a chromatogram, which is a pictorial representation of our separated eluents. Chromatograms. These are charts of the eluents' concentration vs time as they exit the stationary phase. Chemical analysis uses chromatography, a laboratory technique, to separate a mixture into its component parts. The stationary phase is a material that is fixed in a system, and the mobile phase, which is a liquid solvent, dissolves the mixture and carries it through that system.
What are the colours of chromatogram and its main purpose?The various pigments are visible in the chromatogram. Carotene appears brilliant yellow, chlorophyll an is blue-green, chlorophyll b is yellow-green, and xanthophyll is pale yellow-green. (Only two of these pigments may be visible.)
Chromatography's goal is to separate the different components of a mixture. Applications range from a simple examination of a compound's purity to a thorough dissection of its components.
To know more about Chromatogram visit:
https://brainly.com/question/12008770
#SPJ4
What scientist said we cannot predict an exact position of
atoms.
Answers
Answer:
The Heisenberg uncertainty principle states that the exact position and momentum of an electron cannot be simultaneously determined. Warner Heisenberg contributed to atomic theory through formulating quantum mechanics in terms of matrices and in discovering the uncertainty principle, which states that a particle's position and momentum cannot both be known exactly.
Explanation:
i hoped this help
Which of the following statements is/are correct?
I.
II.
Mass of 1 atom of Carbon-12 is equal to 1 amu
Mass of 4 atoms of Carbon-12 is equal to mass of 3 atoms of Oxygen-16
(A) Only I
(B) Only II
(C) Both I and II
(D) None of them
Answers
Answer:
The correct statement is;
(B) Only II
Explanation:
I) The atomic mass unit or amu which is also known as daltons which is defined as 1/12 × the mass of a carbon-12 atom, which is therefore, 1.660538921 × ⁻²⁴ grams
Therefore, the mass of 1 atom of carbon-12 = 12 amu
II) The mass of one mole of oxygen-16 is 16 grams while the mass of one mole of carbon-12 is 12 grams
The mass of 4 atoms of carbon 12 = 4 × 12 amu = 48 amu
The mass of 1 atom of oxygen-16 = 15.99491461956 amu
Therefore, the mass of 3 atoms of oxygen-16 = 3 × 15.99491461956 = 47.9847438587 amu ≈ 48 amu
Therefore only II is correct.
what do you call the corrosive buildup of black powder residue?
Answers
Answer:
Fouling
Explanation:
cccccc Its called Fouling
The corrosive buildup inside the barrel reduces accuracy and this buildup of black powder residue is called fouling.
What is black powder?Gunpowder or black powder can be described as the earliest known chemical explosive. It consists of carbon, sulphur, and potassium nitrate. In the black powder, Sulphur and carbon act as fuels while the saltpeter acts as an oxidizer.
Gunpowder is used as a propellant in artillery, firearms, rocketry, and pyrotechnics, including as a blasting agent for explosives in mining, building pipelines, and road building.
Gunpowder is a low explosive due to its relatively slow decomposition rate and low brisance. Ignition of the black powder packed behind a projectile creates sufficient pressure to force the shot from the muzzle at high speed. It makes a good propellant but is less useful for shattering rock with its low-yield explosive power. Therefore, it is replaced by smokeless powder due to relative inefficiency.
Learn more about Gunpowder, here;
https://brainly.com/question/14367766
#SPJ5
Copper (II) nitrate and sulfuric acid react to form copper (II) sulfate and nitric acid. Of 3.27 moles of copper (II) nitrate react, how many moles of nitric acid will be formed in this reaction
Answers
If 3.27 moles of copper (II) nitrate react, 3.27 moles of nitric acid will be formed in this reaction.
The balanced chemical equation for the reaction between copper (II) nitrate (Cu(NO3)2) and sulfuric acid (H2SO4) is:
3 Cu(NO3)2 + 2 H2SO4 → 3 CuSO4 + 2 HNO3
From the balanced equation, we can see that the molar ratio between copper (II) nitrate and nitric acid is 3:2. This means that for every 3 moles of copper (II) nitrate, 2 moles of nitric acid are produced.
Given that we have 3.27 moles of copper (II) nitrate, we can calculate the number of moles of nitric acid formed using the molar ratio:
(3.27 moles Cu(NO3)2) x (2 moles HNO3 / 3 moles Cu(NO3)2) = 2.18 moles HNO3
Therefore, if 3.27 moles of copper (II) nitrate react, 2.18 moles of nitric acid will be formed in this reaction.
In the reaction between copper (II) nitrate and sulfuric acid, if 3.27 moles of copper (II) nitrate are used, 2.18 moles of nitric acid will be produced according to the stoichiometry of the balanced chemical equation.
To know more about nitric acid, visit:
https://brainly.com/question/22698468
#SPJ11
What type of reaction is 2O3 ----> 3O2?
Answers
Answer:
Explanation:
It is a decomposition although it does not look like it. What is happening is that the O3 is giving up one oxygen, and the oxygen on the right is taking on in the form of a new molecule.
Where is the current North magnetic Pole?
Answers
Answer:
The current location of the North Magnetic Pole is in the Arctic Ocean, near the coast of northern Canada, specifically in the region of northern Quebec and Ellesmere Island in the Canadian Arctic Archipelago.
Explanation:
However, it's important to note that the North Magnetic Pole is not a fixed point on the Earth's surface, but it is instead constantly moving. The North Magnetic Pole is moving away from Canada and towards Siberia at a rate of about 55 km (34 miles) per year. This movement is caused by changes in the Earth's magnetic field and is known as the "wandering" of the magnetic poles. The exact location of the North Magnetic Pole can be determined by regular measurements and it is used as a reference point for navigation.