SHOW WORK
What volume of concentrated nitric acid (15.8 M) is needed to prepare 4.0 L of a 2.0 M solution?
Answers
M
a
= 6.77M - the initial molarity (concentration)
V
a
= 15.00 mL - the initial volume
M
b
= 1.50 M - the desired molarity (concentration)
V
b
= (15.00 + x mL) - the volume of the desired solution
(6.77 M) (15.00 mL) = (1.50 M)(15.00 mL + x )
101.55 M mL= 22.5 M mL + 1.50x M
101.55 M mL - 22.5 M mL = 1.50x M
79.05 M mL = 1.50 M
79.05 M mL / 1.50 M = x
52.7 mL = x
59.7 mL needs to be added to the original 15.00 mL solution in order to dilute it from 6.77 M to 1.50 M......
Also you kinda cute what’s up :)
Related Questions
Please fill in the blanks. 1. Elements: _____ of the _____. 2. Atoms: _____ blocks of all _____. 3. M_____: is______. Made of _____
Answers
Answer:
1.) Elements: building blocks of the universe
2.) Atoms: basic blocks of all matter
3.) Molecules: is matter. Made of atoms
Answer:
Explanation:
1.) Elements: building blocks of the universe
2.) Atoms: basic blocks of all matter
3.) Molecules: is matter. Made of atoms
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture. a) filtered tea. b) freshly squeezed orange juice. c) a compact disc. d) aluminum oxide, a white powder that contains a 2:3 ratio of aluminum and oxygen atoms. e) selenium
Answers
A compact disc is a heterogeneous mixture, filtered tea is a Homogeneous mixture, freshly extracted orange juice is heterogeneous, aluminum oxide is a compound, and selenium is an element.
Is heterogeneous a synonym for abnormal?That is untrue. They really have different meanings and are different terms. A structure that is heterogeneous has pieces or components that are different from one another and that seem irregular or variegated. J
How are things diverse vs homogeneous?The look and content of a homogenous mixture are constant throughout. Solutions are a general term for many homogenous mixes. A heterogeneous mixture is made up of clearly distinct components or stages. The three different states or phases of matter are solid, liquid, and gas.
To know more about Heterogeneous visit :
https://brainly.com/question/11670385
#SPJ4
Which states of matter are present in this sealed container?
A. solid and gas
B. liquid and gas
C. solid and liquid
D. solid, liquid, and gas
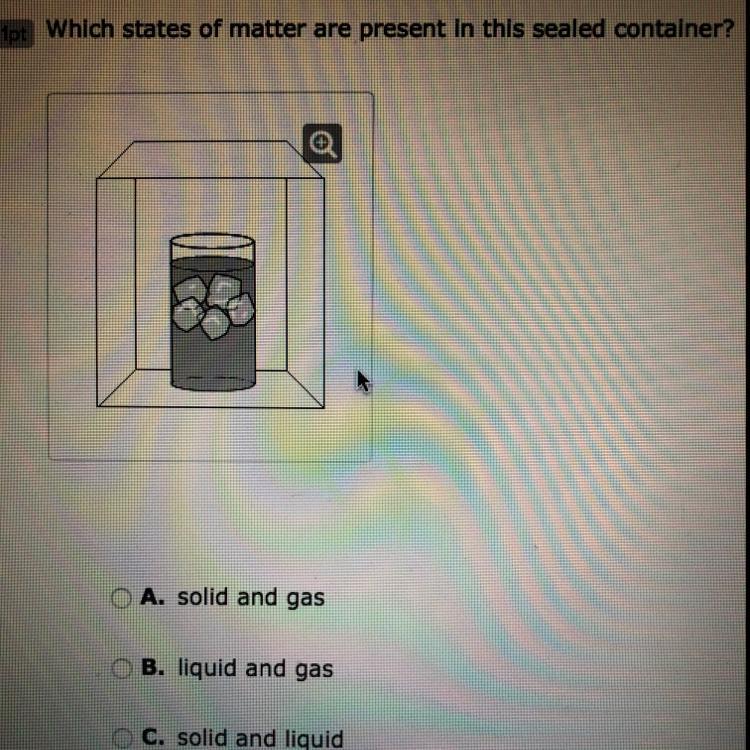
Answers
Answer:
C. solid and liquid
I hope this helps!
which element will have the smallest atomic radius? A. In B. Sn C. Ge D. Ga
Answers
Answer:
According to my opinion The option is C
Explanation:
Because atomic radius decreases across a periond and increase along a group.
in an electrochemical cell, polarization is caused by.?
Answers
Answer:
hydrogen
Explanation:
Polarization is defined as the change in electrode potential of an electrode in an electrochemical cell as electric current is being passed through the cell. It is a kinetic deviation from equilibrium as current is passed through the electrochemical cell.
It is sometimes as a result of the over-potential caused by the accumulation of hydrogen gas at an electrode.
in the distillation of a pure material, why does all of the pure material no vaportize once the boiling point is reched.
Answers
In the distillation of a pure material, all of the pure material not vaporize once the boiling point is reached because more heat would need to be added to the distillate in order to vaporize the liquid from its boiling point.
During distillation, the process of vaporizing a liquid and collecting the resulting vapor as a purified substance, it is important to consider the energy requirements involved.
When a liquid reaches its boiling point, it undergoes a phase change from the liquid phase to the gas phase. This phase change requires the input of energy in the form of heat. The heat breaks the intermolecular forces holding the liquid molecules together, allowing them to transition into the gas phase.
The heat required to vaporize a liquid is not solely determined by the boiling point. The heat required to convert a liquid into a gas is known as the heat of vaporization, and it varies depending on the substance.
When distilling a liquid, such as water, the heat of vaporization must be supplied to convert the liquid into vapor. This energy is absorbed by the liquid, and it is essential to provide continuous heating to maintain the distillation process.
As the liquid is heated and reaches its boiling point, vaporization begins. However, the rate at which the liquid vaporizes depends on the amount of heat being supplied. If the heat input is insufficient, the vaporization process will be slower, and not all of the liquid will vaporize at once.
To ensure the complete vaporization of a liquid during distillation, a sufficient amount of heat must be continuously applied to the system. This allows the heat of vaporization to be continually supplied to the liquid, facilitating the conversion of the entire liquid into vapor.
If the heat input is insufficient, the vaporization process will be slower, and the liquid may not vaporize all at once. Providing adequate and continuous heating is crucial to ensure the complete conversion of the liquid into vapor during distillation.
To know more about distillation here
https://brainly.com/question/31829945
#SPJ4
Based on Lewis structures, predict the ordering of N-O bond lengths in NO+, NO2-, and NO3-
Answers
Based on Lewis structures, the ordering of N-O bond lengths is NO+ < NO2- < NO3-.
In Lewis structures, the number of electron pairs around the central atom can affect the bond lengths. The more electron pairs there are, the greater the repulsion between them, which can lead to longer bond lengths.
In NO+, there are two electron pairs around the central nitrogen atom, resulting in a linear structure. The N-O bond length in NO+ is shorter compared to the other two molecules.
In NO2-, there are three electron pairs around the central nitrogen atom, resulting in a bent structure. The presence of an additional lone pair increases the electron-electron repulsion, leading to longer N-O bond lengths compared to NO+.
In NO3-, there are four electron pairs around the central nitrogen atom, resulting in a trigonal planar structure. The presence of two additional lone pairs further increases the repulsion, resulting in the longest N-O bond lengths among the three molecules.
Based on Lewis structures, the ordering of N-O bond lengths is NO+ < NO2- < NO3-.
To know more about Lewis, visit:
https://brainly.com/question/20300458
#SPJ11
hcn and kcn have similar chemical formulas. however, 0.1 m hcn has a ph of 5.2, while 0.1 m kcn has a ph of 11.2. why do these two compounds behave so differently when they dissolve in water? hcn has a great solubility. hcn is an acid, and kcn is a salt. kcn is much stronger base than hcn. hcn is a nonelectrolyte.
Answers
HCN is highly soluble in water,
With rising temperatures and in extremely saline environments, its solubility declines. HCN is a colorless gas and liquid with an odor reminiscent to bitter almonds, however not everyone can smell it. HCN is created in solution when the cyanide ion interacts with water.
What is HCN?
Prussic acid, also known as hydrogen cyanide, is a chemical substance having the formula HCN and the structural formula HCN. It is a colorless, very deadly, and combustible liquid that boils at 25.6 °C (78.1 °F), only barely over room temperature. Industrial-scale HCN production makes it a highly prized precursor to a wide range of chemical compounds, from medications to polymers. Production of potassium cyanide and adiponitrile, which are used in mining and polymers, respectively, has large-scale uses. Due to its liquid nature, it is more hazardous than cyanide compounds that are solid.
To know more about Prussic acid, visit:
https://brainly.com/question/13326666
#SPJ4
If 7.34 mol of O2 reacts, calculate the grams of CO2 produced.CH4 + 2O2—> CO2 + 2H2O
Answers
Answer:
\(161.48\text{ g}\)Explanation:
Here, we want to get the mass of carbon (iv) oxide produced
From the question, we have the balanced chemical reaction stating that 2 moles of oxygen molecule produced 1 mole of carbon (iv) oxide molecule
The number of moles of carbon (iv) oxide produced from 7.34 mol oxygen is thus:
\(\frac{7.34\times1}{2}\text{ = 3.67 moles}\)1 mole of carbon (iv) oxide contains 44 g
The mass in 3.67 moles will be:
\(44\times3.67\text{= 161.48 g}\)If 250 mL of methane, CH4, effuses through a small hole in 48 s, the time required for the same volume of helium to pass through the hole will be.....?
Answers
If 250 mL of methane (CH4) effuses through a small hole in 48 s, the time required for the same volume of helium to pass through the hole is approximately 96 s.
The effusion rate of a gas is inversely proportional to the square root of its molar mass, according to Graham's law of effusion. In this case, we need to compare the effusion rates of methane and helium.
Since the volume is constant, we can use the ratio of their times of effusion.
Let's assume the molar mass of methane (CH4) is M1 and the molar mass of helium (He) is M2. According to Graham's law, the ratio of the effusion times is given by:
(time for methane) / (time for helium) = √(M2 / M1)
Given that the time for methane is 48 s, we need to find the time for helium. Rearranging the equation, we have:
(time for helium) = (time for methane) / √(M2 / M1)
By substituting the molar masses of methane (16.04 g/mol) and helium (4.00 g/mol), we can calculate:
(time for helium) = 48 s / √(4.00 g/mol / 16.04 g/mol)
(time for helium) = 48 s / √(0.25)
(time for helium) = 48 s / 0.5
(time for helium) = 96 s
Therefore, the time required for the same volume of helium to pass through the hole is approximately 96 seconds.
Learn more about Graham's law of effusion here:
brainly.com/question/30982581
#SPJ11
The energy required to remove an electron from the outermost shell is called
Answers
Answer:
Ionization energy
Explanation:
Ionization energy can be defined as the energy needed to remove an electron from the outermost she'll.
How to identify polar molecules
Answers
Answer:
Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out
Explanation:
C5H12() + 502(9)=
5CO2(g) + 6H2(9)
A. Decomposition
B. Combustion
C. Neutralization
D. Precipitation
Answers
What is the pH of a solution that has a [OH-] = 5.8 x 10^-9
A. 8.2
B. 5.8
C. 9.8
D. 4.2
Answers
The pH of a solution that has a [OH-] = 5.8 x 10⁻⁹ is 5.76.
What is pH?
A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14. Range of 0-6 represents acids and range of 8-14 represents bases. The value of pH 7 represents neutral solution.
pH = -log [H+]
Also, pH = 14 - pOH
Given,
[OH-] = 5.8 x 10⁻⁹
pOH = -log [OH-]
pOH = 9 - 0.763 = 8.23
Hence, pH = 14 - 8.23 = 5.76
Therefore, the pH of a solution that has a [OH-] = 5.8 x 10⁻⁹ is 5.76.
Learn more about pH, here:
https://brainly.com/question/15289741
#SPJ1
How many grams of LiBr are needed to make 1.5 L of a 3.0 M solution?
Answers
Answer: 391 g
Explanation:
For this problem, we need to know that molarity is. Molarity is moles of solute/liters of solution. it is also denoted as M=n/V, which is also mol/L. We are given that the molarity is 3.0 M and the liter is 1.5 L. All we have to do is plug in 3.0 and 1.5 into our formula and solve for moles.
\(3.0M=\frac{n}{1.5L}\)
\(n=4.5 mols\)
Now that we have moles, we can convert moles to grms by using the molar mass of LiBr.
\(4.5 mols*\frac{86.844 g}{1 mol} =391g\)
When might Accurate length measurement be important?
Answers
Answer:
When you are going to measure small lengths or objects or when you are going to measure things with great accuracy.
Explanation:
Three categories of factors that cause illness or injury are shown here. Match each example on the right to its correct match on the
left.
A baby is born with an abnormal number of chromosomes.
Angela was badly injured in a car accident after her air bag
exploded.
Alex forgot to get a flu shot and missed two weeks of school
with the flu. Answer choices pathogenic agents, genes , environmental factors
Answers
Answer:
Alex forgot to get a flu shot and missed two weeks of school
Which color (frequency) of light travels the fastest in a vacuum?
Answers
In a vacuum, all colors (frequencies) of light travel at the same speed, known as the speed of light (c), which is approximately 299,792 kilometers per second (km/s) or 186,282 miles per second (mi/s). Light is an electromagnetic wave, and its speed is independent of its frequency or wavelength in a vacuum. This means that whether the light is red, green, blue, or any other color in the visible spectrum, it will always travel at the same constant speed in a vacuum.
The difference in the speed of light for various colors becomes noticeable when light passes through a medium other than a vacuum, such as air or water. In this case, the speed of light is affected by the refractive index of the medium. Different colors of light have different refractive indices, leading to different speeds and causing the phenomenon known as dispersion, which can be observed in a rainbow.
In summary, in a vacuum, all colors of light travel at the same constant speed, regardless of their frequency. It is only when light passes through a medium with a refractive index that the speed of various colors of light may differ.
to know more about speed of light click this link
brainly.com/question/1555553
#SPJ11
how many protons (p) and neutrons (n) are in an atom of barium-130?
Answers
Answer:
56 protons and 74 neutrons
Explanation:
There are 56 protons and 74 neutrons in an atom of barium-130.
I am pretty sure it's correct, I checked it over a couple of times. If I'm wrong, feel free to correct me!
how would you explain that after putting in the same amount of heat, the two materials have different temperatures?
Answers
Answer:
one material is probably a conductor of heat, which means it absorbs it much better and faster while the other material isn't a conductor and it's taking time to heat up
What is the measure of radioactive decay rate?
alpha emission
beta emission
gamma emission
half life
Answers
Half life, as stated, is a measurement of the rate at which radioactive material decays.
What is radioactive, and what kinds are there?This process can be artificially produced by people, such as within a nuclear reactor, but can also occur spontaneously in nature. Depending on the particles or energy generated during the reaction, there are many kinds of radioactivity. Alpha particles, beta particles, and gamma rays are the three categories.
Briefing:Average and half-life are two characteristics that may be used to describe the decay constant. Moments are used as the measuring unit in both scenarios. The average lifespan of such an element, as indicated by its name, may be expressed in the form of the following affirmation:
Nt=N₀ * e^(−λt).
The duration of time that is defined by how long it takes for half of a material to degrade is known as its half-life (both radioactive and non-radioactive elements). All through process of decay, its rate of decay is constant. It may be seen by:
Nt=N₀* (1/2)^(t/t₁₂).
To know more about Radioactive visit:
https://brainly.com/question/1770619
#SPJ1
Question 8
2 pts
(02.02 LC)
Which of these events leaves small pieces of rocks in new places?
O Deposition
O Weathering
O Erosion
O Oxidation
Answers
Answer:
The correct answer is A.
Deposition
state boyl"s law and Charle's law
Answers
Answer:
hey mateee
Boyle's law :- the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature.
Boyle's law can also be formularized as P1V1 = P2V2
Charle's law :- the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant.
Charle's law can also be formularized as V1/T1 = V2/T2
The melting point of a benzophenone is depressed by 2. 3°c when 0. 358 g of solute is dissolved in 7. 544 g of benzophenone. What is the molar mass of the solute?.
Answers
The formula Molar mass of solute = mass of solute/number of moles of solute can be used to determine the molar mass of a solute if its mass is known.
Solve the problem?Avogadro's number (6.022 x 1023) of molecules (or formula units) make up one mole of a substance (ionic compound).
The mass of 1 mole of a chemical is indicated by its molar mass.
It provides you with the amount of grams per mole of a substance, to put it another way.
calculating the solute's mass
Concentration in g/dm = mass of solute in g
0.358/3= 0.119
Volume in dm = 3.
A sodium chloride solution has a 10 g/dm concentration.
3.
Concentration in g/dm = mass of solute in g
Volume in dm = 3.
3\s = 10 g/dm
0.358× 2 dm 3
To learn more about molar mass refer
https://brainly.com/question/21334167
#SPJ4
At constant pressure and 25^0C , a sample of gas occupies 4.5lit. at what temperature will the gas occupy 9.0lit.
Answers
Answer:
Final temperature = 149 K
Explanation:
Given data:
Initial temperature = 25°C (25+273 = 298 K)
Initial volume = 4.5 L
Final temperature = ?
Final volume = 9.0 L
Solution:
The given problem will be solve through the Charles Law.
According to this law, The volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure.
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
T₂ = T₁V₂/V₁
T₂ = 4.5 L × 298 K / 9.0 L
T₂ = 1341 L K / 9.0L
T₂ = 149 K
Which of the following is a scientific question?
O A. During what time period was the best music composed?
O B. In what year will our team win the championship?
O C. When was the Declaration of Independence written?D. How frequently do earthquakes occur in San Francisco?
Answers
Answer:
D. How frequently do earthquakes occur in San Francisco?
calculate the molar concentration of h3o ions and the ph of the following solutions: (a) 25.0 cm3 of 0.144 m hcl(aq) was added to 25.0 cm3 of 0.125 m naoh(aq), (b) 25.0 cm3 of 0.15 m hcl(aq) was added to 35.0 cm3 of 0.15 m koh(aq), and (c) 21.2 cm3 of 0.22 m hno3(aq) was added to 10.0 cm3 of 0.30 m naoh(aq).
Answers
The pH of a solution is defined as the negative logarithm to the base 10 of the value of the hydronium ion concentration in moles per litre. If the pH is less than 7, then it is acidic and if it is greater than 7, then it will be basic.
1 cm³ = 0.001 L
a) Moles of HCl = 0.144 M × 0.025 L = 3.6 × 10⁻³
Moles of NaOH = 0.125 × 0.025 = 3.125 × 10⁻³
The balanced chemical equation is:
HCl + NaOH → NaCl + H₂O
Moles of HCl remain unreacted is:
3.6 × 10⁻³ - 3.125 × 10⁻³ = 4.75 × 10⁻⁴
Molar concentration of HCl = 4.75 × 10⁻⁴ / 0.05 = 9.5 × 10⁻³ M
pH = - log [9.5 × 10⁻³] = 2.02
b) Moles of HCl = 0.00375
Moles of NaOH = 0.00525
Moles of NaOH remain unreacted is:
0.00525 - 0.00375 = 0.0015
Molar concentration of NaOH = 0.0015 / 0.025 = 0.06 M
pOH = - log [OH⁻] = -log [ 0.06] = 1.22
pH = 14 - 1.22 = 12.78
[H₃O⁺] = 10⁻pH = 1.65 × 10⁻¹³
c) Moles of HCl = 0.22 × 0.0212 = 0.0046
Moles of NaOH = 0.30 × 0.01 = 0.003
Moles of HCl remain unreacted is:
0.0046 - 0.003 = 0.0016
Molar concentration of HCl = 0.0016 / 0.0312 = 0.0512
pH = - log [0.0512] =1.290
To know more about pH, visit;
https://brainly.com/question/31447199
#SPJ4
Determine the empirical formula of a
compound containing 2.6444g of gold
and 0.476g of chlorine.
Answers
Answer:
AuCl
Explanation:
Given parameters:
Mass of Gold = 2.6444g
Mass of Chlorine = 0.476g
Unknown:
Empirical formula = ?
Solution:
Empirical formula is the simplest formula of a compound. Here is the way of determining this formula.
Elements Au Cl
Mass 2.6444 0.476
Molar mass 197 35.5
Number of moles 2.6444/197 0.476/35.5
0.013 0.013
Divide by the
smallest 0.013/0.013 0.013/0.013
1 1
The empirical formula of the compound is AuCl
In Lab, We Used Oxone To Oxidize Borneol To Camphor. Write Chemical Equations Describing The Reaction Of NaCl And Oxone.
Answers
Answer:
NaO2SO3 -> Na2SO4 + O2
Explanation:
The reaction of NaCl (sodium chloride) and Oxone (potassium peroxymonosulfate) is described by the following chemical equations:
The first step in the reaction is to dissociate oxone into its component ions:
Oxone (potassium peroxymonosulfate) -> K+ (potassium ion) + O2SO3- (peroxymonosulfate ion)
The peroxymonosulfate ion (O2SO3-) then oxidizes the sodium chloride (NaCl) to produce sodium peroxymonosulfate (NaO2SO3) and chlorine gas (Cl2):
NaCl + O2SO3- -> NaO2SO3 + Cl2
The sodium peroxymonosulfate (NaO2SO3) then decomposes into sodium sulfate (Na2SO4) and oxygen gas (O2):
NaO2SO3 ) -> Na2SO4 + O2.#SPJ4
.
what are the factors affecting gravity?
Answers
Gravity, as a fundamental force of nature, is influenced by several factors. The following are some of the key factors affecting gravity:
Mass: The most significant factor affecting gravity is the mass of the objects involved. According to Newton's law of universal gravitation, the gravitational force between two objects is directly proportional to the product of their masses. Greater mass leads to a stronger gravitational force.Distance: The distance between two objects also plays a crucial role in the strength of gravity. According to the inverse square law, the gravitational force decreases as the distance between objects increases. As objects move farther apart, the gravitational attraction between them weakens.Gravitational Constant: The gravitational constant, denoted by G, is a fundamental constant in physics that determines the strength of the gravitational force. It is a universal constant and does not change, affecting the overall magnitude of gravity.Shape and Distribution of Mass: The distribution of mass within an object can influence the gravitational field it generates. Objects with a more compact and concentrated mass distribution will have a stronger gravitational pull compared to those with a more spread-out mass distribution.External Influences: Gravity can be influenced by external factors such as nearby celestial bodies or the presence of other forces. For example, the gravitational interaction between the Earth and the Moon affects tides on Earth's surface.