Answers
Answer:
1.46g of PbCrO₄ are the theoretical yield
Explanation:
Theoretical yield is defined as the maximum amount of products that could be produced (Assuming a yield of 100%).
The reaction of Lead (II) nitrate with sodium chromate is:
Pb(NO₃)₂(aq) + Na₂CrO₄(aq) → PbCrO₄(s) + 2NaNO₃ (aq)
First, we need to find molar mass of each reactant in order to determine limiting reactant (As the reaction is 1:1, the reactant with the lower number of moles is the limiting reactant). The moles of the limiting reactant = moles of Lead (II) chromate (The precipitate):
Moles Pb(NO₃)₂ -Molar mass: 331.21g/mol-
1.50g * (1mol / 331.21g) = 4.53x10⁻³ moles Pb(NO₃)₂
Moles Na₂CrO₄ -Molar mass: 161.98g/mol-
1.75g * (1mol / 161.98g) = 0.0108 moles
Pb(NO₃)₂ is limiting reactant and moles of PbCrO₄ are 4.53x10⁻³ moles. The mass is:
4.53x10⁻³ moles PbCrO₄ * (323.19g / mol) =
1.46g of PbCrO₄ are the theoretical yieldRelated Questions
embalming authorization may be given by all of the following except the? Bailee, next of kin, spouse, or decedent
Answers
The correct answer is "decendent." Embalming authorization may be given by the bailee, next of kin, or spouse, but not by the decedent.
When is the authorization given?
The term "decedent" refers to the person who has died; they are unable to give consent for embalming or any other post-mortem procedures. According to legal and cultural customs, the choice to embalm is often taken by the designated representative, such as the bailee (person in charge of the body), next of kin, or spouse.
Hence, embalming authorization may be given by the bailee, next of kin, or spouse, but not by the decedent.
Learn more about embalming:https://brainly.com/question/30163437
#SPJ1
C6H12O6 + 602 → 6CO2 + 6H₂O
The most efficient ratio is
1 C6H12O6 6 02.
Which set of reactants will be the most
efficient (least wasteful of materials) for
the reaction?
A. 1.0 mol C6H12O6 and 3.0 mol O₂
B. 1.5 mol C6H₁2O6 and 3.0 mol O₂
C. 3.0 mol C6H₁2O6 and 6.0 mol O₂
D. 0.5 mol C6H₁2O6 and 3.0 mol O₂
Answers
Answer:
D
Explanation:
The ratio of C6H12O6 (which will be referred to as "the carb") to oxygen is 1 to 6, so if we find an answer which has the same ratio, it should be chosen. A is 1:3
B is even worse with a ratio of the carb to oxygen of 1:2
C is the same as B, 1:2
D has a ratio of the carb to oxygen of 1:6, which is what we are looking for.
List the 2 pKa's for H2SO4
Answers
La función de la levadura en quimica
Answers
Explanation:
las levaduras son pequeños organismos unicelulares que se alimentan de azúcares simples y los descomponen en dióxido de carbono, alcohol (etanol, específicamente), moléculas de sabor y energía. El proceso se conoce como fermentación.
what is the mass of 9.3 x 10^ 24 molecules of glucose, C6H12O6 (C6,H12,O6; 180.18 g/mol

Answers
Molecular mass of 9.3 x 10^ 24 molecules of glucose = 1675.6 x 10^ 24
It is given that the mass of glucose is 180.18 g/mol
so if we have to calculate the mass of 9.3x 10^ 24 molecules of glucose
we will apply a simple unitary method i.e,
9.3 x 10^ 24 * 180.18 g/mol = 1675.67 x 10^ 24
To calculate the molecular mass of a molecule, multiply the subscript (number of atoms) by the atomic mass of each element in the molecule and add those masses together.
Remember that - To determine the compound's molecular mass in grams per mole, use the molecular formula.
Divide the supplied mass by the molar mass of the chemical to convert it to moles.
By dividing the number of moles by Avogadro's number, you may convert from moles to molecules.
To learn more about molecular mass please visit -
https://brainly.com/question/18446366
#SPJ1
Complete the sentence.
Atoms form chemical bonds to satisfy the ___________rule and to become _______.
Answers
Answer:
Atoms form chemical bonds to satisfy the octet rule and to become stable.
1. In general, what is meant by the term chemical bond? Name and
describe the 3 principle types of chemical bonds.
Answers
Answer: The simplest and most common type is a single bond in which two atoms share two electrons. Other types include the double bond, the triple bond, one- and three-electron bonds, the three-center two-electron bond and three-center four-electron bond. ... Bonds within most organic compounds are described as covalent.
Explanation:
Scientists launch a rocket, and they monitor its acceleration and the force exerted by its engines. As the rocket gets higher, the monitors show that the acceleration of the rocket is increasing but the force exerted stays the same. How do Newton’s laws explain why the scientists could expect this to happen?
Answers
The force applied to the rocket by its engines remains constant as it moves up, while its mass decreases, resulting in an increase in acceleration.
Newton's laws of motion provide an explanation for the acceleration of a rocket as it moves away from the ground. According to Newton's second law, the force exerted on an object is directly proportional to its acceleration, and the force required to move an object increases as its mass increases.
In the case of a rocket, its mass decreases as it consumes fuel, which means that less force is required to move it as it climbs higher into the atmosphere.
As the rocket moves up, its acceleration increases while the force exerted on it remains constant. Newton's second law of motion explains that the acceleration of an object is directly proportional to the force applied to it. According to the second law of motion, an object's acceleration is equal to the force exerted on it divided by its mass.
This means that as the rocket climbs higher and its mass decreases due to the consumption of fuel, less force is required to accelerate it, and so its acceleration increases. In other words, the rocket's acceleration is increasing because the force required to move it is decreasing due to the decreasing mass of the rocket.
This phenomenon is also related to Newton's third law of motion, which states that every action has an equal and opposite reaction. The force exerted by the rocket's engines is balanced by an equal and opposite force exerted on the rocket by the exhaust gases, according to this law.
For more such questions on acceleration visit;
https://brainly.com/question/26590057
#SPJ8
In a titration, 25.0 of dilute sulfuric acid was used to react completely with 20.0 of 0.400 mol/ aqueous sodium hydroxide.
2 NaOH (aq) + (aq) → (aq) + 2 (1) (a)Calculate the number of moles of sodium hydroxide that reacted.
(b) Calculate the number of moles of sulfuric acid that reacted with the sodium hydroxide. (c) What is the concentration of sulfuric acid in g/
Answers
Answer:
Explanation:
The balanced chemical equation for the reaction between sulfuric acid and sodium hydroxide is:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
From the equation, we can see that one mole of sulfuric acid reacts with two moles of sodium hydroxide. Therefore, the number of moles of sodium hydroxide used in the reaction is:
n(NaOH) = c(NaOH) x V(NaOH) = 0.400 mol/L x 0.0200 L = 0.00800 mol
Since one mole of sulfuric acid reacts with two moles of sodium hydroxide, the number of moles of sulfuric acid used in the reaction is twice that of sodium hydroxide:
n(H2SO4) = 2 x n(NaOH) = 2 x 0.00800 mol = 0.0160 mol
The concentration of the sulfuric acid can be calculated by dividing the number of moles by the volume used in the titration:
c(H2SO4) = n(H2SO4) / V(H2SO4) = 0.0160 mol / 0.0250 L = 0.640 M
Therefore, the concentration of the dilute sulfuric acid is 0.640 M.
Why the gross reading is needed when doing the titration?
Answers
Answer:
The answer is below
Explanation:
Because various factors can affect the actual value of the titration outcome. Some of these factors can range from errors from a researcher also known as human error, misreading the quantities, researcher's perception of the color, or wrong procedure in carrying out the experimentation.
Hence, in order to avoid such error, a researcher needs to be thorough during the process of experimentation, and using gross reading can help to avoid these errors when the titre value is eventually determined.
A car with a mass of 2000 kg accelerates at a rate of 3 m/s2. How much force does this car have?
Answers
Answer:
6000N
Explanation:
f= ma
f= 2000kg×3mls2
f=6000N
Answer:
6000 N
Explanation:
Given,
Mass= 2000 kg
Acceleration= 3 m/s²
Force= ?
Force= mass* acceleration
Force= 2000* 3 = 6000 N
Therefore, the force possessed by the car is 6000 N
Calculate the percent composition of Ca3P2
Answers
Answer:
Ca - 66%, P - 34%
Explanation:
So, this is the formula we can use to find the amount of each element:
Element count * Atomic mass = Mass
Plug in our elements for this:
Ca - 3*40.078=120.234
P - 2*30.973=61.946
Now, to find the percentage of mass, we must find total mass, and divide the two elements mass count by this total mass:
120.234+61.946=182.18
Now divide each element mass by the total mass:
Ca - 120.234/182.18=0.6599(Round to 0.65)
P - 61.946/182.18=0.34002(Round to 0.34)
Then multiply both numbers by 100 to get the percentage:
Ca - 65%
P - 34%
So these our your two answer!
Hope this helps!
what is 1.01 km to mm in scientific notation
Answers
Answer:
= 3.456 × 1011
Explanation:
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
Write and balance the equation for the combustion of the fatty acid lauric acid, (C12H24O2)
.
Express your answer as a chemical equation. Identify all of the phases in your answer.
Answers
The combustion of lauric acid is shown as CH3(CH2)10COOH(s) + 18O2(g) -----> 12H20(l) + 12CO2(g)
What is combustion?In a combustion reaction, a substance is burnt in oxygen. If the substance is an organic compound a the case is here, the products are carbon dioxide and hydrogen.
No the equation of the combustion of lauric acid is shown as;
CH3(CH2)10COOH(s) + 18O2(g) -----> 12H20(l) + 12CO2(g)
Learn more about combustion:https://brainly.com/question/15117038?
#SPJ1
Balance the following half-reaction in acid (some electrons, e, will be needed to balance it):Fe²+ ——-> FeO4²-
Answers
The balanced half-reaction is Fe²⁺ + 2e⁻ --------> FeO₄²⁻
The given reaction is a redox reaction. Balancing a redox reaction is a bit different than solving a normal chemical reaction. The first step is to find the oxidation state of each element in the reactant as well as the product side. By doing so we get,
The oxidation state of Fe - +2
On the product side, we get,
The oxidation state of Fe - +6
The oxidation state of O - -2
The next step is to calculate the difference between the oxidation states.
The difference in oxidation state of Fe - +6 - (+2) = +4
The difference in oxidation state of O - 0 -2 = -2
To balance this reaction, 2 electrons should be added to the reactant side so that the oxidation states of O and Fe get balanced equally. By doing so we get,
Fe²⁺ + 2e⁻ --------> FeO₄²⁻
This is a balanced half-reaction in acid
To know more about redox reactions, click below:
https://brainly.com/question/21851295
#SPJ9
an element with the valence electron configuration 3s2 3p6 belongs to period

Answers
Answer:
The answer is D
Explanation:
The element is in the 3rd period because n = 3.
Why would a table be more suitable than a graph to show data
Answers
Tables are more suitable than a graph to show data because they present data in as close to raw form as possible.
How can data be represented?Data can be represented in Tables, charts and graphs. These data representation are used for two broad purposes.
The first is to support the collection, organization and analysis of data as part of the process of a scientific study. The second is to help present the conclusions of a study to a wider audienceUnlike the case of graphs that use abstraction to focus on trends and numerical relationships, tables present data in as close to raw form as possible.
Tables are usually meant to be read, so they are ideal when the data that is present are those type of data that cannot easily be presented visually, or when the data requires more specific attention.
Learn more about tables at: https://brainly.com/question/12151322
#SPJ1
What is the average atomic mass of 10 hydrogen -1 molecules?
Answers
Answer:
1.674 x 10^-23 grams
Explanation:
Hydrogen-1 is called Protium
wikipedia
atomic mass of Protium is 1.00794 amu
sciencedirectcom
atomic mass of 10 Protiums is 10.0794 amu
10.0794 amu in grams is
1.6737236x10^-23 grams
Which is an example of plasmas in nature?
Answers
Answer: nbhhvyyvub
Explanation:
please help with all 4, i don’t understand it
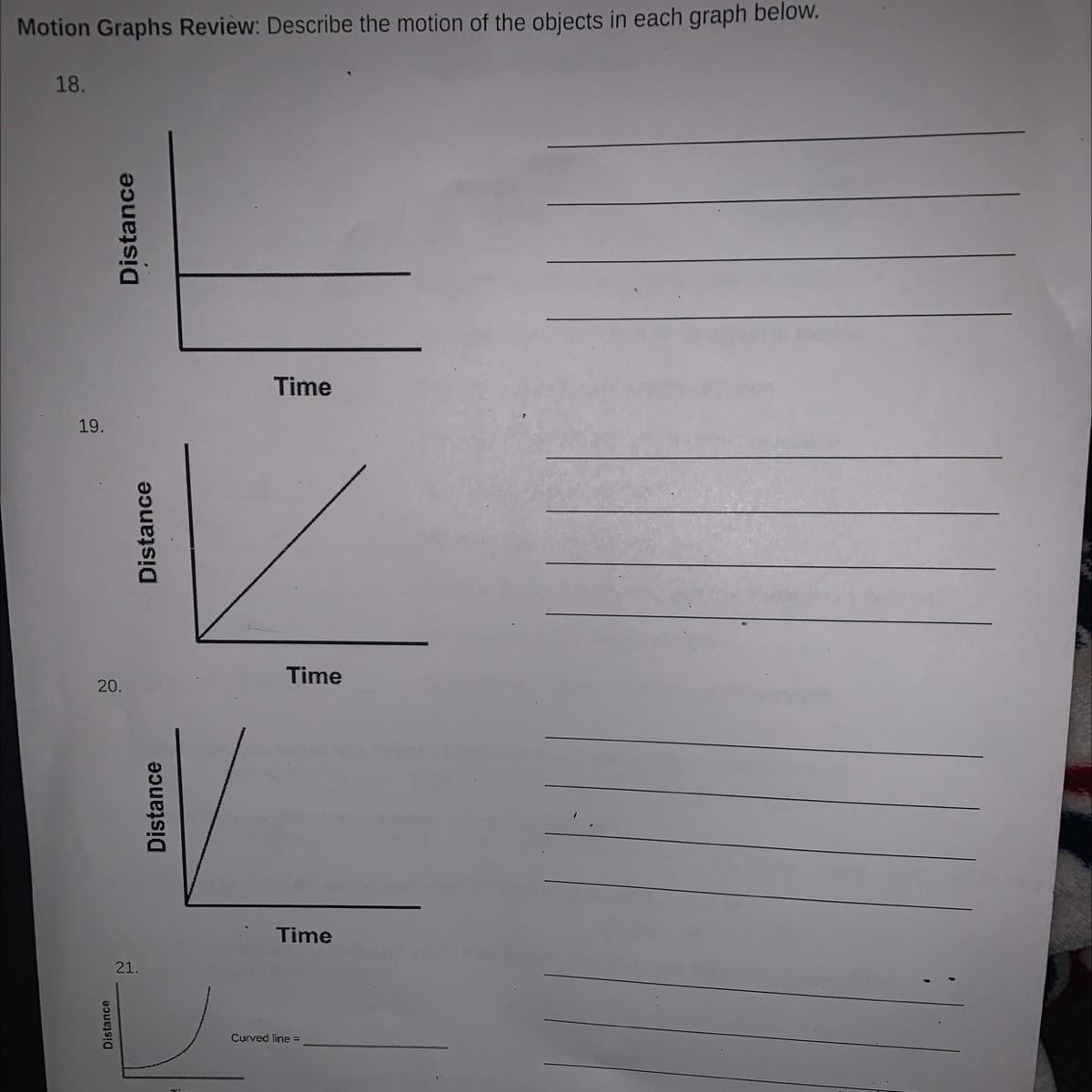
Answers
Answer:
1. the distance of the object is staying the same over time
2. the distance of the object is increasing over time
3. the distance of the object is increasing rapidly
4. the object started gaining distance slowly then began to rapidly increase in distance over time
Hey duckers
Pp poopoo everyday

Answers
Answer:
So when a cat puts up its middle finger its usually trying to scare you off.
Explanation:
it's gonna kill you.
WILL BE MARKED BRAINLIEST IF U ANSWER CORRECT
PLUS 30 PT
Why does DNA dissolve in water?
Why is salt added to solution of DNA and water?
How does ethanol help the precipitate form?
After a pellet is formed, is the DNA in the pellet or the supernate?
Answers
Explanation:
Because of this, DNA and RNA can easily dissolve in water. ... Adding ethanol helps the Na+ and PO3- ions come together, because ions travel easier in ethanol than water. The supernate is removed and new ethanol is added in the second washing. This removes any residual salt that remained on the pelleted DNA.
Answer:
DNA is polar due to its highly charged phosphate backbone. Its polarity makes it water-soluble (water is polar) according to the principle "like dissolves like". ... This fact makes water a very good solvent for charged compounds like salts.
Your DNA's sugar phosphate backbone is charged. By adding salt, we help neutralize the DNA charge and make the molecule less hydrophilic, meaning it becomes less soluble in water. The salt also helps to remove proteins that are bound to the DNA and to keep the proteins dissolved in the water.
It is well known that Ethanol has a lower dielectric constant than water, making it to promote ionic bond formations between the Na+ (from the salt) and the PO3- (from the DNA backbone), further, causing the DNA to precipitate.
Explanation:
Ideally DNA needs to be precipitated with pellet and should not remain in supernatant. DNA is acidic in nature and therefore, needs optimum salt concentration in the buffer to be pelleted from a solution. At very low salt concentration or without salt DNA would remain in supernatant.
types of reactions lab report
Answers
Answer:
There are five main types of chemical reactions. These are synthesis, decomposition, combustion, single replacement and double replacement.
Explanation:
Hope Its helps you ! : )What is the volume of a 0.5 M solution of HCl if it contains 36.5 grams of solute?
Molar mass (H -1 g/mol)(Cl - 35.5 g/mol)
___ L (Answer Format: X)
Answers
Answer: 2 L or 2000 mL
Explanation:
A 1 molar (1 M) solution is equal to 1 mole of the solute dissolved in 1 L of solution.
For HCl (mw 36.5) 1 mole = 36.5 g
1 molar (1 M) = 1 mole/1 liter (or 36.5 g/L)
So
0,5 M = 1 mole/x
(x is the volume we are solving for)
Multiply both sides by x and you get
0.5x=1
Now multiple both sides by 2
X=2
So it’s 2L volume
The fluorocarbon compound C2Cl3F3 has a normal boiling point of 47.6 ∘C. The specific heats of C2Cl3F3(l) and C2Cl3F3(g) are 0.91 J/g⋅K and 0.67 J/g⋅K, respectively. The heat of vaporization for the compound is 27.49 kJ/mol.
Part A
Calculate the heat required to convert 75.0 g of C2Cl3F3 from a liquid at 13.60 ∘C to a gas at 76.00 ∘C.
Answers
The heat required is to convert 75.0 g of C₂Cl₃F₃ from a liquid at 13.60 ∘C to a gas at 76.00 ∘C is 17.55 kJ.
To solve this problem, we need to consider the different steps involved in the process of converting 75.0 g of C₂Cl₃F₃ from a liquid at 13.60 ∘C to a gas at 76.00 ∘C;
Heating the liquid C₂Cl₃F₃ from 13.60 ∘C to its boiling point at 47.6 ∘C, Vaporizing the liquid C₂Cl₃F₃ at its boiling point, and heating the resulting gas from 47.6 ∘C to 76.00 ∘C
Now, we can use the equations to calculate the heat required for each step;
q₁ = m × C₁ × ΔT₁
where q₁ is the heat required, m is the mass of C₂Cl₃F₃, C₁ is the specific heat of C₂Cl₃F₃(l), and ΔT₁ is the temperature change from 13.60 ∘C to 47.6 ∘C.
q₁ = 75.0 g × 0.91 J/g⋅K × (47.6 ∘C - 13.60 ∘C)
= 2466 J
q₂ = n × ΔHvap
where q₂ is the heat required, n is the number of moles of C₂Cl₃F₃, and ΔHvap is the heat of vaporization of C₂Cl₃F₃.
n = m/M
= 75.0 g / 137.37 g/mol
= 0.5464 mol
q₂ = 0.5464 mol × 27.49 kJ/mol
= 15.038 kJ
q₃ = m × C₂ × ΔT₂
where q₃ is the heat required, m is the mass of C₂Cl₃F₃(g), C₂ is the specific heat of C₂Cl₃F₃(g), and ΔT₂ is the temperature change from 47.6 ∘C to 76.00 ∘C.
m = n × M
= 0.5464 mol × 137.37 g/mol
= 75.0 g
q₃ = 75.0 g × 0.67 J/g⋅K × (76.00 ∘C - 47.6 ∘C)
= 1446 J
The total heat required is the sum of the heats required for each step;
\(q_{total}\)= q₁ + q₂ + q₃
= 2466 J + 15.038 kJ + 1446 J
= 17.55 kJ
Therefore, the total heat required is 17.55 kJ.
To know more about heat required here
https://brainly.com/question/14679329
#SPJ1
explain why different objects appear to be different colors.
Answers
Answer:
Different objects appear to be different colors, because we see color by light bouncing off an object and reflecting or transmitting into our eye. The amount of light that reflects or transmits into our eye depend on the color that we will see.
Paragraph
Styles
Just Lemons Lemonade Recipe Equation:
2 water + sugar + lemon juice - 4 lemonade
Mole conversion factors:
1 mole of water = 1 cup - 236.598
1 mole of sugar = 1 cup - 1982
1 mole of lemon juice = 1 cup - 229.96 €
1 mole of lemonade = 1 cup = 225.285
Analysis Questions
1. Based on taste observations only, which ingredients were in excess in the lemonade samples in
Activity One?
2. Based on the data in Activity Two, which excess ingredients are affecting the taste of the lemonade in
the sample batch?
3. What can Just lemons, Inc. do during production to reduce the amount of excess ingredients and
improve the taste of their lemonade?
4. Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three
starting ingredients. Show your stoichiometric calculations below.
5. During factory inspection, Just Lemons, Inc. discovered that a water valve to the lemonade mixing
station was not functioning. Once they repair it, more water will enter the mixing station. From what
you know about the limiting and excess ingredients for current lemonade production, what advice
would you give engineers about the upcoming increase in water?

Answers
Based on limiting and excess reactant, having an appropriate stoichiometric ratio will help maximize the quality of lemonade made by Just lemons Inc.
Making LemonadeLemonade is made using the following ingredients in the equation below:
2 water + sugar + lemon juice - 4 lemonadeWhich ingredient will affect the taste?Since both sugar and lemon juice have tastes, an excess of either of the two will affect the taste of lemonade.
Which excess ingredient is affecting the lemonade in sample batch?Since this is an activity, the ingredient affecting the lemonade will either be sugar or lemon juice.
What can Just lemons, Inc. do during production to reduce the amount of excess ingredients and improve the taste of their lemonade?Just lemons incorporated can acquire and use more accurate measurement equipments.
They can also employ a skilled technician.
What can be done to reduce the amount of leftover ingredients?The amount of leftover ingredients can be reduced by using an appropriate stoichiometry ratio of reactants.
What can be done by engineers about upcoming increase in water?Limiting reactants are used up in a reaction while excess reagents are leftover.
Either water, lemon juice or sugar is a limiting reactant.
Therefore, it would be best the engineers determine the limiting reactant so water will not be in excess.
Therefore, based on limiting and excess reactant, having an appropriate stoichiometric ratio will help maximize the quality of lemonade made by Just lemons Inc.
Learn more about limiting reactant and excess reactant at: https://brainly.com/question/14222359
is this correct?............................................................................................................................................
............................................................................................................................................
...........................................................................................................................................

Answers
3.09 g is the theoretical mass of AlBr₃(s) produced.
How to setup dimensional analysis?The following dimensional analysis setup could be used to determine the theoretical mass of AlBr₃(s) (molecular mass = 266.69 g/mol) produced based on reacting 84.2 g of a 0.005 mol/L solution of Br₂(l) (density=1019 g/L) with excess Al(s) as described in the following equation:
3Br₂(l) + 2Al(s) → 2AIBr₃(s)
The dimensional analysis setup to calculate the mass of AlBr₃(s) produced is as follows:
84.2 g Br₂ (l) × (1 L solution / 1019 g Br₂(l)) × (0.005 mol Br₂(l) / 1 L solution) × (2 mol AlBr₃(s) / 3 mol Br₂(l)) × (266.60 g AlBr₃(s) / 1 mol AlBr₃(s)) = 3.09 g AlBr₃(s)
Therefore, the theoretical mass of AlBr₃(s) produced is 3.09 g.
Find out more on dimensional analysis here: https://brainly.com/question/24514347
#SPJ1