"Henry mixed salt and water together in a cup until he observed a clear solution. He measured the mass of the solution. Then he placed the cup outside for several sunny days during the summer. After a week, he observed that only solid salt remained in the cup and the mass had decreased. Henry concluded that a physical and chemical change occurred in this investigation."
Which statements correctly defend or dispute his conclusion?
A.)He is correct. Dissolving salt in water is a physical change, but evaporating the water is a chemical change. Formation of a solid is evidence that a chemical change occurred.
B.)He is correct. Evaporation is a physical change, but dissolving salt in water is a chemical change. The change in mass is evidence that a chemical change occurred.
C.)He is incorrect. Dissolving salt in water and evaporation of the water are both physical changes. The reappearance of salt is evidence that the change was reversible by a physical change, so it could not be a chemical change.
D.)He is incorrect. Dissolving salt in water and evaporation of the water are both chemical changes. The reappearance of salt is evidence that the change was reversible by a chemical change, so it could not be a physical change.
Answers
Answer:
C.)He is incorrect. Dissolving salt in water and evaporation of the water are both physical changes. The reappearance of salt is evidence that the change was reversible by a physical change, so it could not be a chemical change.
Explanation:
From the analogy of the problem presented, we can see that Henry is grossly incorrect. His conclusion from the process of the experiment he carried out is completely wrong.
Physical changes are changes that alters the physical properties of matter particularly the form and state.
Chemical changes leads to the formation of a new kind of matter.
We can see that since the salt was obtained back after evaporation, no change has occurred to it.
Therefore, evaporation in itself is a physical change process.
Answer:
c on ed
Explanation:
Related Questions
Use this graphic to explain how matter is conserved in a nuclear reaction

Answers
Nuclear processes alter the sorts of atoms present, but chemical reactions do not. The electrons in the atom play a significant role in chemical reactions when nucleus reactions occur in atom nuclei.
What is nuclear explain?The energy found in an atom's nucleus, or core, is known as nuclear energy. Energy maintains the nucleus of atoms together, the minuscule units that make up all matter in the universe. The dense nucleus of an atom has an enormous quantity of energy.
Why is a nuclear threat a threat?The most lethal weapons on earth are nuclear weapons. One can wipe out an entire metropolis, perhaps killing millions of people, endangering the ecosystem and the lives of future generations due to its long-term devastating impacts.
To know more about Nuclear visit:
https://brainly.com/question/18187269
#SPJ1
1.
At constant pressure. 50 milliliters (mL) of a gas
at 20°C is heated to 30° C. The new volume of
the gas in milliliters (ml) is equal
Answers
Answer:
\(\boxed {\boxed {\sf V_2=75 \ mL}}\)
Explanation:
Since the pressure is constant, the only variables we need to work with are temperature and volume. We will use Charles's Law, which states the volume of a gas is directly proportional to the temperature. The formula is:
\(\frac{V_1}{T_1}=\frac{V_2}{T_2}\)
Originally, the gas was 50 milliliters at 20 degrees celsius. Substitute these values into the left side of the equation.
\(\frac{50 \ mL}{20 \textdegree C}=\frac{ V_2}{T_2}\)
We don't know the volume of the new gas, but we know the temperature was changed to 30 degrees celsius.
\(\frac{50 \ mL}{20 \textdegree C}=\frac{ V_2}{30 \textdegree C}\)
Since we are solving for the new volume, we must isolate the variable. It is being divided by 30 °Cand the inverse of division is muliplication. Multiply both sides by 30 °C.
\(30 \textdegree C*\frac{50 \ mL}{20 \textdegree C}=\frac{ V_2}{30 \textdegree C}* 30 \textdegree C\)
\(30 \textdegree C*\frac{50 \ mL}{20 \textdegree C}= V_2\)
The units of degrees celsius cancel, so we are left with milliliters as the units.
\(30*\frac{50 \ mL}{20}= V_2\)
\(\frac{1500 \ mL}{20}= V_2\)
\(75 \ mL=V_2\)
The new volume of the gas is 75 milliliters.
Compared to an atom of c-12 an atom of c-14 has a greater.
Answers
An atom of C-14 has the same number of protons as an atom of C-12, but a greater mass number due to the presence of more neutrons.
An atom of carbon has six protons and its atomic number is six. An atom of C-12, a stable isotope of carbon, has six neutrons while an atom of C-14, an unstable isotope of carbon, has eight neutrons. Thus, an atom of C-14 has a greater mass number than an atom of C-12 due to the presence of more neutrons, even though they have the same number of protons and atomic number.
An atom's atomic number is the number of protons present in its nucleus, whereas its mass number is the number of protons plus the number of neutrons. Since C-14 has the same number of protons as C-12 but more neutrons, it has a greater mass number than C-12, while the number of electrons in both atoms is the same. As a result, the correct answer is that compared to an atom of C-12, an atom of C-14 has a greater mass number.
Learn more about isotope here:
https://brainly.com/question/21536220
#SPJ11
Complete question is:
Compared to an atom of c-12 an atom of c-14 has a greater
number of electrons
number of protons
atomic number
mass number
LOT OF POINTS!!!! PLEASE HELP ASAP NEED. HELP ASAP NO ROCKY PLS RN I WILL GIVE BRAINLIEST
Identify one or more choices that best complete the statement or answer the question.
Formulas of pairs of compounds of nitrogen oxides are given below. Which pairs are consistent for a given mass of nitrogen such that the first compound contains twice the mass of oxygen as the second compound?
a. NO4:NO2
b. NO4:N2O
c. NO2:NO
d. NO:N2O
a., b., and d. only
a. and c. only
a., c., and d. only
c. and d. only
Answers
a 30.00-ml sample of 0.125 m hcooh is being titrated with 0.175 m naoh. what is the ph after 30.0 ml of naoh has been added? ka of hcooh
Answers
The pH after 30.0 ml of NaOH has been added is 2.18.
To find the pH after 30.0 ml of 0.175 M NaOH has been added to a 30.00 ml sample of 0.125 M HCOOH, you need to use the balanced chemical equation for the reaction between HCOOH and NaOH:
HCOOH + NaOH → NaCOOH + H2O
This equation shows that 1 mole of HCOOH reacts with 1 mole of NaOH, so the number of moles of NaOH added to the HCOOH solution is:
n(NaOH) = C(NaOH) x V(NaOH) = 0.175 mol/L x 0.0300 L = 0.00525 mol
Since the stoichiometry of the reaction is 1:1, this means that 0.00525 mol of HCOOH has been neutralized by the NaOH. The remaining amount of HCOOH is:
n(HCOOH) = C(HCOOH) x V(HCOOH) - n(NaOH) = 0.125 mol/L x 0.0300 L - 0.00525 mol = 0.00225 mol
Now you can use the Ka expression for HCOOH to find the concentration of H+ ions:
Ka = [H+][COO-]/[HCOOH] = 1.8 x 10^-4
[H+][0.00225]/[0.1225 - 0.00525] = 1.8 x 10^-4
[H+] = 0.00659 M
pH = -log[H+] = 2.18
To learn more about balanced chemical equation click here
brainly.com/question/15052184
#SPJ11
Calculating volume (formula) and density of regular shaped objects
Please help I need to complete this assignment fast :( I’m not sure on how to do it, If you don’t know how to do it don’t answer pls
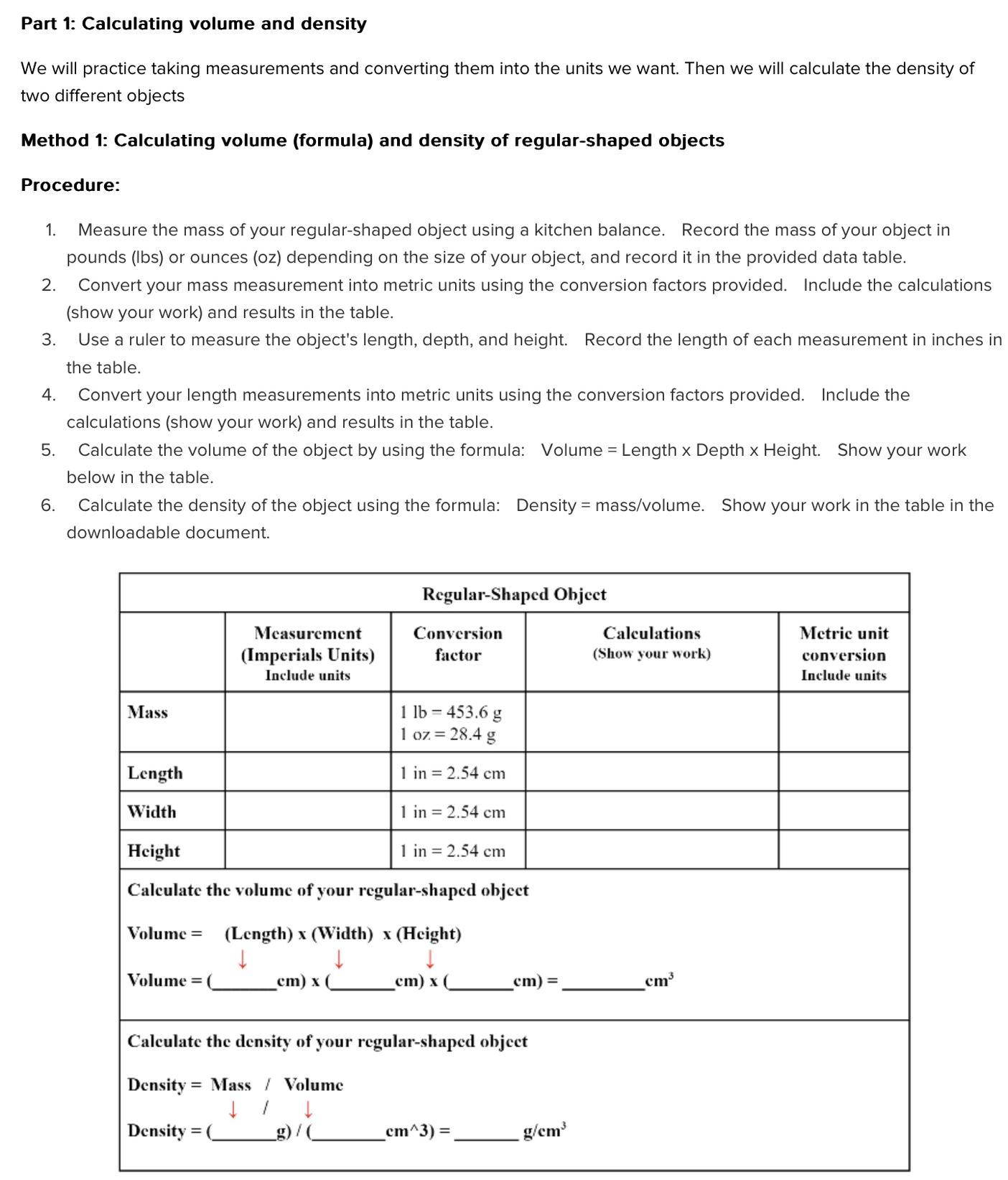
Answers
The density of the unknown sample is 1.025 g / mL and its salt composition is 3.55 %.
How to solve
PART A: Density of a regular shaped object:
Trial 1: mass of the object = 162.20 g
volume of object = L x H x W = 4.90 cm x 3.90 cm x 2.90 cm
= 55.419 cm^3
Therefore density of the object = mass / volume = 162.20 g / 55.419 cm^3
= 2.9268 g/cm^3
trial 2: mass of the object = 162.18 g
volume of object = L x H x W = 4.89 cm x 3.90 cm x 2.88 cm
= 54.92448 cm^3
Therefore density of the object = mass / volume = 162.18 g / 54.92448 cm^3
= 2.9528 g/cm^3
Average = [ 2.9268 + 2.9528 ] /2 = 5.8796 / 2 = 2.9398 g / cm^3 = 1.94 g / cm^3.
The accepted value is 2.73 g / cm^3 for aluminium. The difference is 0.21
% error = 100 x difference / accepted value = 100 x 0.21/2.73 = 7.7 %.
---------------------------------------------------------------------------------------------------
Part B: Determination of density of an irregular shaped object:
Trial 1:
mass of the marble chips = 10.25 g
Volume of the marble chip = final volume of water - initial volume of water
= 53.8 - 50 = 3.8 mL
Therefore density of marble chip = mass / volume = 10.25 g / 3.8 mL
= 2.697 g / mL
Trial 2:
mass of the marble chips = 10.32 g
Volume of the marble chip = final volume of water - initial volume of water
= 53.9 - 50.1 = 3.8 mL
Therefore density of marble chip = mass / volume = 10.32 g / 3.8 mL
= 2.716 g / mL
Average = [2.697 + 2.716] / 2 = 5.413 / 2 = 2.71 g / mL
The accepted density of marble chip = 2.70 g / mL The difference is 0.01
% error = 100 x difference / accepted value = 100 x 0.01/ 2.70 = 0.37 %.
--------------------------------------------------------------------------------------------------------------------
PART C: Determination of density of saline solution:
Trial 1:
Volume of the saline solution = 10 mL
mass of the saline solution = finall mass - initial mass
= 35.66 - 25.36 = 10.3 g
Density of the saline solution = mass / volume = 10.3 g / 10 mL = 1.03 g / mL
Trial 2:
Volume of the saline solution = 10 mL
mass of the saline solution = finall mass - initial mass
= 35.55 - 25.35 = 10.2 g
Density of the saline solution = mass / volume = 10.2 g / 10 mL = 1.02 g / mL
Average =[ 1.03 + 1.02 ] / 2 = 1.025 g / mL
Thus the unknown sample B has the density of 1.025 g / mL.
The composition of salt in this solution can be determined by interpolation.
salt % = 0 + 5 x [ 1.025-0.998] / [1.036 - 0.998] ( using the values given in the table )
= 0 + 5 x 0.027 / 0.038
= 3.55 %.
Thus the density of the unknown sample is 1.025 g / mL and its salt composition is 3.55 %.
Read more about density here:
https://brainly.com/question/6838128
#SPJ1
As the term "peroxide" is used in Chapter 10, it can refer to which structure(s)? A) ROOR B) CH2OOCH O o RCOOLR c) RCOOCR D) Two of these choices. E) Three of these choices. ОЕ OD OC
Answers
As the term "peroxide" is used in Chapter 10 it can refer to RCOOCR structure.
Is peroxide harmful to the body?Hydrogen peroxide solutions up to 9% in concentration are often safe when consumed; nonetheless, even a 3% solution is slightly irritating to nasal mucosa and might result in vomiting and diarrhea. Industrial-strength solutions (10%) when consumed induce systemic toxicity and have been linked to deaths.
Is hydrogen peroxide the same as peroxide?The fact that oxygen is an anion and hydrochloric acid is a chemical substance is the main distinction between the two peroxides. The specific class of oxygen compounds known as peroxides has distinctive characteristics. Thenard, a French chemist, made the discovery of hydrogen peroxide in 1818.
To know more about peroxide visit:
https://brainly.com/question/29102186
#SPJ4

Base your answers to questions 1 and 2 on
the diagram below, which represents a solid material
of uniform composition.
2.0 cm
Density-2.7 g/cm³
-20 cm-
We
1. The mass of this piece of material is approximately
A) 32 g
Q4.4g
X) less
-3.0 cm
2. When this material is placed in a container of water, i
sinks to the bottom of the container. Compared to the
density of water, the density of this material is
B) greater
the same
B) 0.23 g
9.3 g

Answers
Answer:
A. 32 g
Explanation:
Density = 2.7 g/cm3
Volume = 2 x 2 x 3 = 12 cm3
Mass = dV = 2.7 x 12 = 32.4 g or 32 g
If the half life of a radioactive isotope is 1 day, then how much of the original isotope remains at the end of two days?
(a) 50%
(b) 100%
(c) Zero
(d) 25%
(e) 12.5%
Answers
what is a galvanic cell made of?
A. two electrodes in electrolyte solution
B. two beakers connected by a tube
C. two ceramic plates in pure water
D.two medal strips surrounded by air
Answers
Explanation:
A because A galvanic cell consists of two half-cells, such that the electrode of one half-cell is composed of metal A, and the electrode of the other half-cell is composed of metal B; the redox reactions for the two separate half-cells are thus: An+ + ne− ⇌ A.
Answer:
A
Explanation:
PLEASE ANSWER NOW!!!
A train travels 200 miles at an average speed of 50 miles per hour. How long does it take the train to travel this distance?
Answers
Answer:
The answer is 100 m/p/h because you divide the total 200 miles and the 50 speed and you get about 100 m/p/h
Explanation:
What is the relationship between mole, Avogadro number and mass?
a.1mole=6.022 × 1023 atoms and molecules= g atomic/molecular mass
b. 1mole =6.022 × 1023 atoms and molecules /1 g atomic/molecular mass
c. 2mole=6.022 × 1023 atoms and molecules=1 g atomic/molecular mass
d. 1mole < 6.022 × 1023 atoms and molecules=1 g atomic/molecular mass
Answers
Answer:
The mass of one mole of a substance is equal to that substance's molecular weight. ... water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams. ... Avogadro's number is a proportion that relates molar mass on an atomic ... one molecule of water (H2O), one mole of oxygen (6.022×1023 of O atoms)
(PLSSS HEELP)Which landform is created by wind?
Delta
Desert gravel
Horns
Oxbow lake
Answers
This Example Illustrates Gasoline Blending Problems Faced In A Petroleum Refinery. We Need To Blend Gasoline From Three
Answers
Gasoline blending in petroleum refineries involves analyzing the properties of different components and determining the optimal mixing ratios to produce gasoline that meets specific octane rating and quality requirements.
Gasoline blending is a critical process in petroleum refineries where different components are combined to produce the desired gasoline product. In this example, the challenge is to blend gasoline from three different components.
To solve the gasoline blending problem, various factors need to be considered such as the desired octane rating, volatility, and environmental regulations. The first step is to determine the optimal proportion of each component based on their individual characteristics. This involves analyzing the properties of each component, such as its research octane number (RON), motor octane number (MON), and vapor pressure.
The second step is to develop a blending strategy that achieves the desired gasoline specifications. This involves determining the appropriate mixing ratios of the three components to meet the target octane rating and other quality requirements. The blending process requires precise calculations and adjustments to ensure the final gasoline product meets the desired specifications.
Additionally, economic considerations play a role in gasoline blending. The cost of each component and the market demand for specific gasoline grades can influence the blending decisions. Refineries aim to optimize the blend to minimize costs while meeting quality standards.
Learn more about Gasoline blending here:
https://brainly.com/question/13719873
#SPJ11
a sample of a molecular compound was analyzed and found to contain 0.707 grams carbon (c), 0.2372 grams of hydrogen (h). determine the empirical formula of the compound. given the added information that the molar mass of the compound is 8 times the empirical mass, determine the molar mass of the compound.
Answers
The molar mass of the compound is 128.4 g/mol.
The empirical formula is an empirical formula that represents the lowest whole-number ratio of the atoms present in a compound. The empirical formula for the molecular compound is calculated using the percentage composition of the elements present in the compound. The steps used to find the empirical formula are as follows:
Find the mass of each element present in the compound.Convert each mass to moles.Divide each mole value by the smallest number of moles.Round to the nearest whole number and write the subscripts.The molar mass is the mass of a substance that contains 6.02 × 10²³ atoms or molecules. To calculate the molar mass of a compound, add the masses of all the atoms present in the compound.
C=0.707g,12.01 g/mol=0.0588 molCnH=0.2372 =1.01g/m=0.235 mol H
nH=4nC
The empirical formula of the compound is CH4. The molar mass of the compound can be calculated using the empirical formula.
M=12.01 g/mol+4(1.01 g/mol)=16.05 g/mol
The molar mass of the compound is 8 times the empirical mass, so the actual molar mass is;
M=8(16.05{g/mol})=128.4g/mol. The molar mass of the compound is 128.4 g/mol.
More on molar mass: https://brainly.com/question/15583536
#SPJ11
what is one example of a electromagnetic wave
Answers
Answer:
Examples of EM waves are radio waves, microwaves, infrared waves, X-rays, gamma rays, etc.
Answer:
Electromagnetic waves can be split into a range of frequencies. This is known as the electromagnetic spectrum. Examples of EM waves are radio waves, microwaves, infrared waves, X-rays, gamma rays, etc.
Explanation:
11.0 kJ are used to melt 55.0 grams of copper at its melting point. Calculate the heat of fusion of copper.
Answers
Answer:
Explanation:
a substance's enthalpy of fusion tells you how much heat is needed in order to convert
1 g
of said substance from solid at its melting point to liquid at its melting point.
In water's case, an enthalpy of fusion equal to
333.55 J g
−
1
tells you that
1 g
of ice at
0
∘
C
can be converted to
1 g
of liquid water at
0
∘
C
by supplying
333.55 J
of heat.
Your ice cube has a mass of
55.0 g
, which means that it will require
55.0
g
⋅
=
Δ
H
fus
333.55 J
1
g
=
18,345.25 J
Rounded to three sig figs, the number of sig figs you have for the mass of the ice cube, the answer will be
heat needed
=
∣
∣
∣
∣
¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯
a
a
18,300 J
a
a
∣
∣
−−−−−−−−−−
describe the basic assumptions of the kinetic molecular theory of gases that confirm the ideal behavior of gases.
Answers
The kinetic-molecular principle of gases assumes that perfect gasoline molecules are constantly moving.
have negligible quantity;have negligible intermolecular forces undergo flawlessly elastic collisions have a median kinetic strength proportional to the suitable fuel's absolute temperature.The gasoline debris has a negligible extent. The gas debris is similarly sized and no longer has intermolecular forces (enchantment or repulsion) with other fuel particles. The gasoline debris flows randomly in settlement with Newton's legal guidelines of motion. The gasoline debris has perfect elastic collisions without energy loss.
Learn more about kinetic molecular theory here:-https://brainly.com/question/134712
#SPJ4
draw the structures of the three primary (1°) amines with molecular formula c5h13n that contain five carbon atoms in a continuous chain.
Answers
Here are the structures of the three primary amines with molecular formula C5H13N that contain five carbon atoms in a continuous chain:
Structure 1: 1-Aminopentane
Structure 2: 2-Aminopentane
Structure 3: 3-Aminopentane
To draw the structures of the three primary amines with molecular formula C5H13N that contain five carbon atoms in a continuous chain, we first need to determine the possible ways of arranging the functional group NH2 on a 5-carbon chain.
Aliphatic amines with one amino group and one hydrocarbon group less than the corresponding alcohol are called primary amines. We can arrange the functional group NH2 in three ways on a 5-carbon chain:
On carbon 1
On carbon 2
On carbon 3
The three primary amines with the molecular formula C5H13N are as follows:
Structure 1: N attached to carbon 1 (1-aminopentane)
Structure 2: N attached to carbon 2 (2-aminopentane)
Structure 3: N attached to carbon 3 (3-aminopentane)
Here are the structures of the three primary amines with molecular formula C5H13N that contain five carbon atoms in a continuous chain:
Structure 1: 1-Aminopentane
Structure 2: 2-Aminopentane
Structure 3: 3-Aminopentane
learn more about primary amines here
https://brainly.com/question/31711034
#SPJ11
A 100.0-mL aliquot of 0.200 M aqueous potassium hydroxide is mixed with 100.0 mL of 0.200 M
aqueous magnesium nitrate.
(a) Write a balanced chemical equation for any reaction that occurs.
(b) What precipitate forms?
(c) What mass of precipitate is produced?
(d) Calculate the concentration of each ion remaining in solution after precipitation is complete.
i mostly need help on the last one
Answers
The balanced equation of the reaction is:
2 KOH (aq) + Mg(NO₃)₂ ---> Mg(OH)₂ (s) + 2 KNO₃ (aq)The precipitate formed is magnesium hydroxide.
The mass of precipitate produced is 0.58 g.
The concentration of the ions remaining in the solution are:
[Mg²⁺] = 0.05 M
[NO₃⁻] = 0.1 M
What is the balanced equation of the reaction of aqueous potassium hydroxide and aqueous magnesium nitrate?The balanced equation of the reaction of aqueous potassium hydroxide and aqueous magnesium nitrate is given below:
2 KOH (aq) + Mg(NO₃)₂ ---> Mg(OH)₂ (s) + 2 KNO₃ (aq)
Magnesium hydroxide is obtained as a precipitate in the reaction above.
The mass of precipitate produced is calculated as follows:
moles of KOH (aq) reacting = 0.200 * 100 / 1000 = 0.02 moles
moles of Mg(NO₃)₂ reacting = 0.2 * 100 / 1000 = 0.02 moles
From the equation of reaction, KOH is the limiting reagent.
Moles of precipitate formed = 0.02 / 2 = 0.01 moles
molar mass of Mg(OH)₂ (s) = 58 g/mol
Mass of precipitate formed = 0.01 * 58
Mass of precipitate formed = 0.58 g
Moles of Mg(NO₃)₂ remaining = 0.02 - 0.01 = 0.01 moles
total volume of solution = 200 mL or 0.2 L
The concentration of each ion remaining in the solution is as follows:
[Mg²⁺] = 0.01 / 0.2 = 0.05 M
[NO₃⁻] = 0.01 * 2 / 0.2 = 0.1 M
Learn more about concentration at: https://brainly.com/question/23437000
#SPJ1
6. Mia and her best friend Claudia are comparing eye colors. Mia has blue eyes, and Claudia has brown eyes. What is a difference in eye color among the same species called?
A. chromosomes
B. traits
C. variation
D. mutation
Answers
Answer:
D
Explanation:
Luck
In an ecosystem, the difference in eye color among the same species is called variation.
What is an ecosystem?
Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.There are different ecosystems present in the environment.
Learn more about ecosystem,here:
https://brainly.com/question/1061425
#SPJ5
research the composition of both compact and spongy bone and describe your findings. note the minerals and proteins that make up this tissue.
Answers
Compact Bone is composed of mineralized matrix and bone cells. whereas, Spongy Bone consists of a network of trabeculae, which are thin bony spicules or plates interconnected to create a porous framework.
Compact Bone:
Compact bone, also known as cortical bone, forms the outer layer of bone and provides strength and support. It is composed of mineralized matrix and bone cells.The main minerals found in compact bone include hydroxyapatite, which is a crystalline form of calcium phosphate, and calcium carbonate. These minerals contribute to the hardness and rigidity of the bone tissue.
In addition to minerals, compact bone contains several proteins that contribute to its structure and function. Collagen is the predominant protein found in the bone matrix. It provides flexibility and tensile strength to the bone, allowing it to resist breaking under stress. Other proteins, such as osteocalcin and osteopontin, are involved in mineralization processes and regulate bone remodeling.
Spongy Bone:
Spongy bone, also called trabecular or cancellous bone, is found at the inner layer of bone and forms a lattice-like structure. It consists of a network of trabeculae, which are thin bony spicules or plates interconnected to create a porous framework. This arrangement provides strength to the bone while keeping it lightweight.
Similar to compact bone, spongy bone contains mineralized matrix and bone cells. The minerals present in spongy bone are also hydroxyapatite and calcium carbonate. However, spongy bone has a higher proportion of spaces within its structure compared to compact bone.
Proteins found in spongy bone include collagen, which provides structural support, and other non-collagenous proteins involved in bone development, remodeling, and mineralization.
Overall, both compact and spongy bone consist of mineralized matrix containing hydroxyapatite and calcium carbonate, along with collagen and other proteins that contribute to the structure, strength, and function of the bone tissue. The specific arrangement and density of these components differ between compact and spongy bone, allowing them to fulfill different roles within the skeletal system.
Learn more about Compact Bone at: https://brainly.com/question/2099742
#SPJ11
Bones are made up of two types of tissue: compact and spongy bone. Compact bone (cortical) forms the hard external layer of all bones while spongy (cancellous) bone forms the inner layer of all bones. Both types of bones are composed of specialized cells, mineral salts, and collagen fibers.
Explanation:Our bones are made up of two types of tissue: compact bone and spongy bone. Compact bone, also known as cortical bone, forms the hard external layer of all bones and surrounds the medullary cavity, or bone marrow. This bone tissue consists of units called osteons or Haversian systems, featuring mineral matrix and living osteocytes connected by canaliculi, which transport blood.
Spongy bone, on the other hand, or cancellous bone, forms the inner layer of all bones. Unlike compact bone tissue, spongy bone tissue does not contain osteons. It consists of trabeculae: lamellae that are arranged like rods or plates. In between these trabeculae, we'll find the red bone marrow that is responsible for forming blood cells.
Both types of bone tissues contain specialized cells, mineral salts (mainly calcium and phosphorus), and collagen fibers. The integration of these minerals and proteins is critical for the robust structure and resilience of the skeletal system.
Learn more about Bone Composition here:https://brainly.com/question/34187890
how much heat is released if a 10.0 gram piece of aluminum is cooled from 70°c to 50°c?
Answers
As a result, the aluminum emits -180 J of heat when it is reduced from 70 to 50 degrees Celsius.
Why does science increase the temperature?The mobility of atoms and molecules is accelerated when energy is added (heating), increasing the temperature. By withdrawing energy (cooling), atoms and molecules slow down and the temp drops as a result. Conduction is a technique that allows energy to be added to or taken away from a medium.
The following formula is used to calculate how much heat is emitted when aluminum is reduced from 70°C to 50°C:
Q = mcΔθ
where;
m is the aluminum's mass.
Specific heat capacity is c.
Δθ is a temperature change.
Q = (10)(0.9)(50 - 70) (50 - 70)
Q = -180 J
To know more about heat visit:
https://brainly.com/question/12473602
#SPJ4
2502(g) + O. (g) = 2S0 (g) + 392 kJ
Determine the amount of heat released by the production of 1. 0 mole of SO3 (g)
Answers
The amount of heat released by the production of 1.0 mole of SO3(g) is 196 kJ.
To determine the amount of heat released by the production of 1.0 mole of SO3(g), we need to first balance the chemical equation:
2SO2(g) + O2(g) = 2SO3(g) + 392 kJ
Now, we can see that 2 moles of SO3 are produced by releasing 392 kJ of heat. To find the heat released for 1 mole of SO3, we can set up a proportion:
(392 kJ) / (2 moles of SO3) = x kJ / (1 mole of SO3)
Solving for x:
x = (1 mole of SO3) * (392 kJ) / (2 moles of SO3)
x = 196 kJ
So, the amount of heat released by the production of 1.0 mole of SO3(g) is 196 kJ.
To learn more about heat, refer below:
https://brainly.com/question/1429452
#SPJ11
The weight of an object and its mass are always the same.
True
False
Answers
Answer:
While the weight of an object varies in proportion to the strength of the gravitational field, its mass is constant, as long as no energy or matter is added to the object. Therefore, false.
Explanation:
Answer:
The correct answer should be false
Explanation:
at 298 k, the value of kc for the reaction h2(g) br2(g) ⇌ 2hbr(g) is 2.0 × 1019. what is kc for hbr(g) ⇌ 1/2h2(g) 1/2br2(g)?
Answers
Answer:
Explanation:
To find the value of Kc for the reaction HBr(g) ⇌ 1/2H2(g) + 1/2Br2(g), we can use the relationship between the equilibrium constants of reverse reactions.
For the given reaction: H2(g) + Br2(g) ⇌ 2HBr(g)
We are given the value of Kc as 2.0 × 10^19 at 298 K.
To find the equilibrium constant for the reverse reaction, we take the inverse of Kc.
Kc(reverse) = 1/Kc(forward)
Kc(reverse) = 1/(2.0 × 10^19)
Kc(reverse) = 5.0 × 10^(-20)
Now, we can use the equilibrium constant expression for the reverse reaction to determine the equilibrium constant for the reaction HBr(g) ⇌ 1/2H2(g) + 1/2Br2(g).
Kc = ([1/2H2][1/2Br2]) / [HBr]
Since the coefficients are halved in the reverse reaction, the equilibrium constant is also squared:
Kc = ([H2]^0.5[Br2]^0.5) / [HBr]
Kc = (√[H2]√[Br2]) / [HBr]
Therefore, the equilibrium constant Kc for the reaction HBr(g) ⇌ 1/2H2(g) + 1/2Br2(g) is approximately 5.0 × 10^(-20).
Learn more about equilibrium concentration: https://brainly.com/question/16203171
#SPJ11
Give the number of protons and the number of neutrons in the nucleus of the following isotopes: a) Carbon-14 b) Cobalt-60 c) Gold-197 d) Uranium-235
Answers
Explanation:
We are given different isotopes and we have to identify the number of protons and neutrons that are in the nuclueus of each atom.
a) Carbon-14:
By definition two isotopes are atoms that have the same atomic number but different mass number. The atomic number of an atom is equal to the number of protons of that atom, and the mass number is equal to the number of protons plus the number of neutrons.
atomic number = n° of protons
mass number = n° of protons + n° of neutrons
n° of protons = atomic number
n° of neutrons = mass number - n° of protons
n° of neutrons = mass number - atomic number
If two isotopes have the same atomic number but different mass number we can say that two isotopes have the same number of protons but different number of neutrons.
In we pay attention to carbon-14 we can look for its atomic number in the period table: 6. And its mass number is the one that we are given after the name of the element: 14.
n° of protons = atomic number = 6
n° of protons = 6
n° of neutrons = mass number - atomic number = 14 - 6
n° of neutrons = 8
b) Cobalt-60:
atomic number = 27 (from the periodic table)
mass number = 60
n° of protons = atomic number = 27
n° of protons = 27
n° of neutrons = mass number - atomic number = 60 - 27
n° of neutrons = 33
c) Gold-197:
atomic number = 79 (from the periodic table)
mass number = 197
n° of protons = atomic number = 79
n° of protons = 79
n° of neutrons = mass number - atomic number = 197 - 79
n° of neutrons = 118
d) Uranium-235:
atomic number = 92 (from the periodic table)
mass number = 235
n° of protons = atomic number = 92
n° of protons = 92
n° of neutrons = mass number - atomic number = 235 - 92
n° of neutrons = 143
Answer:
a) Carbon-14: n° of protons = 6 n° of neutrons = 8
b) Cobalt-60: n° of protons = 27 n° of neutrons = 33
c) Gold-197: n° of protons = 79 n° of neutrons = 118
d) Uranium-235: n° of protons = 92 n° of neutrons = 143
Calculate how many liters of oxygen are produced when 45.0 g of KClO3 decomposes.
Answers
12.33 liters of oxygen are produced when 45.0 g of KClO3 decomposes.
How many liters of oxygen are produced ?The mass in grams of one mole of a material is its molar mass. The molar mass of a material may be found by adding the molar masses of its constituent atoms, as demonstrated in this video. The computed molar mass may then be used to convert between mass and the quantity of moles in the material.
KClO3 ------> kcl+ 3/2 O2
KClO3 122.55 gm in terms of molar mass.
Given mass = 459 moles of KCl03= 45 10.367 moles 122.55,
we know that the reaction produces 1 mole of kuo and 3 moles of O2.
I mole + 3/2 mule 02.. 0.367 mole X mole 02
X= 0.367X3 =0.5505 mol using the straightforward unitary technique.
Oxygen capacity at STP = 0.5505 x 22.41
12.33 liters
12.33 liters of oxygen are produced when 45.0 g of KClO3 decomposes.
To learn more about molar mass refer to:
https://brainly.com/question/837939
#SPJ1
When a car uses a gallon of gas, how much carbon dioxide is emitted into the environment? I give branlyest
Answers
Answer:
19.5
Please tell me if wrong.
When a car uses a gallon of gas, 22.0 miles per gallon of carbon dioxide is emitted into the environment.
What happens when carbon dioxide is emitted?After being released into the atmosphere, carbon dioxide (CO₂) is initially quickly dispersed among the atmosphere, the upper ocean, and plants.
The sources of carbon dioxide emissions include both natural and human activities. Decomposition, oceanic discharge, and respiration are examples of natural sources.
The annual carbon dioxide emissions from a typical passenger automobile are around 4.6 metric tonnes. This estimates that the typical gasoline car on the road today gets roughly 22.0 miles per gallon of fuel and travels about 11,500 miles annually.
Thus, 22.0 miles per gallon of carbon dioxide is emitted into the environment.
To learn more about the carbon dioxide, follow the link;
https://brainly.com/question/3049557
#SPJ6
Which are characteristics of a prokaryotic cell? Select three options. contains DNA lacks DNA contains ribosomes lacks ribosomes contains a nucleus lacks a nucleus
Answers
Answer:
Prokaryotic cells contain DNA, contain ribosomes, and lack a nucleus.
Answer:
Contains DNA
Contains Ribosomes
lacks a nucleus
Explanation: I took the quiz on edge