Answers
3H₂S + 8H⁺ + Cr₂O₇⁻² → 3S + 2Cr⁺³ + 7H₂O this is the balanced chemical equation
What is Balanced Chemical Equation ?The balanced chemical equation is the equation in which the number of atoms on the reactant side is equal to the number of atoms on the product side in an equation.
The given chemical equation is
Cr₂O7+ H₂S → Cr³+S
Now, assign the oxidation number to each atom in the given equation.
\(\overset{+6}{Cr} \overset{-2}{O_7} + \overset{+1}{H} \overset{-2}{S} \rightarrow \overset{+3}{Cr} + \overset{0}{S}\)
Now separate the half reaction and balance the elements other than H and O.
H₂S → S
Cr₂O₇⁻² → 2Cr⁺³
Now, add water to balance oxygen
H₂S → S
Cr₂O₇⁻² → 2Cr⁺³ + H₂O
Now balance hydrogen by adding H⁺
H₂S → S + 2H⁺
14H⁺ + Cr₂O₇⁻² → 2Cr⁺³ + 7H₂O
Now, balance the charge
H₂S → S + 2H⁺ + 2e⁻
6e⁻ + 14H⁺ + Cr₂O₇⁻² → 2Cr⁺³ + 7H₂O
Now, balance the atoms multiply by 3
3 [H₂S → S + 2H⁺ + 2e⁻]
3H₂S → 3S + 6H⁺ + 6e⁻
6e⁻ + 14H⁺ + Cr₂O₇⁻² → 2Cr⁺³ + 7H₂O
Now, add both the half reaction
3H₂S + 6e⁻ + 14H⁺ + Cr₂O₇⁻² → 3S + 6H⁺ + 6e⁻ + 2Cr⁺³ + 7H₂O
Now, simplify the equation
3H₂S + 8H⁺ + Cr₂O₇⁻² → 3S + 2Cr⁺³ + 7H₂O
Thus from the above conclusion we can say that 3H₂S + 8H⁺ + Cr₂O₇⁻² → 3S + 2Cr⁺³ + 7H₂O this is the balanced chemical equation.
Learn more about the Balanced chemical equation here: brainly.com/question/26694427
#SPJ9
Related Questions
Hard question!
Try to help me if u can?
An aqueous solution of zinc nitrate reacts in a double displacement reaction with an aqueous solution of sodium hydroxide to form a precipitate.
A. Write the balance chemical equation for the reaction, including state sign?
B. Write a total ionic equation?
C. Write a net ionic equation?
D. State the spectator ions in the reaction
Answers
Answer:as below
Explanation:Zn(NO3)2 (aq) + 2 NaOH (aq) —> Zn(OH)2 (ppt) + 2NaNO3
Zn++ + 2NO3- + 2Na++ + 2OH- —> 2NO3- + 2Na+ + Zn(OH)2
Zn++ + 2OH- —> Zn(OH)2
spectator ions are Na, NO3
How many moles of nitrogen gas would be produced if 8.65 moles of copper(II) oxide were reacted with excess ammonia in the following chemical reaction? 2 NH₃(g) + 3 CuO (s) → 3 Cu(s) + N₂(g) + 3 H₂O(g)
Answers
6.4875 moles of nitrogen gas would be produced if 8.65 moles of copper(II) oxide were reacted with excess ammonia.
Given the following balanced equation, 2 NH₃(g) + 3 CuO (s) → 3 Cu(s) + N₂(g) + 3 H₂O(g). We are required to determine the number of moles of nitrogen gas that would be produced if 8.65 moles of copper(II) oxide were reacted with excess ammonia. We can use stoichiometry to solve this problem. Stoichiometry is the quantitative relationship between the reactants and products in a chemical reaction. It allows us to make predictions about the amount of product produced or reactant required in a chemical reaction. Stoichiometry relies on the balanced chemical equation for the reaction. In this case, the balanced chemical equation is 2 NH₃(g) + 3 CuO (s) → 3 Cu(s) + N₂(g) + 3 H₂O(g).
The balanced chemical equation shows that 2 moles of ammonia react with 3 moles of copper(II) oxide to produce 1 mole of nitrogen gas and 3 moles of water. This means that the mole ratio of ammonia to nitrogen gas is 2:1. We can use this mole ratio to determine the number of moles of nitrogen gas produced in the reaction. We know that 8.65 moles of copper(II) oxide is reacted with excess ammonia. Since copper(II) oxide is the limiting reagent, we can use it to calculate the number of moles of ammonia used in the reaction. The molar ratio of copper(II) oxide to ammonia is 3:2. Therefore, we can calculate the number of moles of ammonia used in the reaction as follows:Number of moles of ammonia = (3/2) × number of moles of copper(II) oxideNumber of moles of ammonia = (3/2) × 8.65Number of moles of ammonia = 12.975 molesWe know that the mole ratio of ammonia to nitrogen gas is 2:1. Therefore, the number of moles of nitrogen gas produced in the reaction is half the number of moles of ammonia used.Number of moles of nitrogen gas produced = (1/2) × number of moles of ammoniaNumber of moles of nitrogen gas produced = (1/2) × 12.975Number of moles of nitrogen gas produced = 6.4875 moles.
for such more questions on moles
https://brainly.com/question/29367909
#SPJ8
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
Select the correct terms to complete this statement about charged particles.
Like charges attract | repel, and opposite charges attract repel. According to Coulomb's law, as the distance between two charged particles decreases, the force between the particles decreases I increases. As the magnitude of the charges decreases, the force decreases | increases.
Answers
Like charges repel each other, while opposite charges attract each other. This principle is one of the fundamental aspects of electrostatics. According to Coulomb's law, the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
As the distance between two charged particles decreases, the force between them increases. This is because the closer the particles are, the stronger the electric field they create, leading to a stronger force of interaction.
On the other hand, as the magnitude of the charges decreases, the force between the particles also decreases. This is because the force is directly proportional to the product of the charges. If one or both of the charges are smaller, the force they exert on each other will be weaker.
In summary, according to Coulomb's law, decreasing the distance between charged particles increases the force between them, while decreasing the magnitude of the charges decreases the force. This understanding of the relationship between charge, distance, and force is crucial in explaining the behavior of charged particles and the interactions between them.
Know more about Coulomb's law here:
https://brainly.com/question/26892767
#SPJ8
Vinegar is sold at the grocery store with a concentration of 5.0 % acetic acid. How many grams of acetic acid are in 28 g of Vinegar?
Answers
White vinegar typically consists of 93%–96% water and 4–7% acetic acid. It can be used to cooking, bake, cleaning, and get rid of weeds. It can also help you lose weight and lower your blood sugar and cholesterol. Consumption is safe in moderation, but excessive consumption or when combined with certain medications could be harmful.
Apple cider vinegar is widely used in cooking and as a salad dressing because it contains acetic acid and nutrients like vitamins C and B vitamins. But at the same time, it's been utilized customarily as medication. It helps in losing weight.
Learn more about vinegar, here:
https://brainly.com/question/23700611
#SPJ1
Hydrogen 1, hydrogen 2, hydrogen 3
If you had to choose, which one would you use/for what purpose?
Answers
Answer:
Hydrogen-2 has one neutron, hydrogen-1 has none.
Explanation:
A scientist that studies earthquakes is known as a?
Answers
Answer:
Seismologists
Explanation:
Big brain
(b) Washing soda crystals react with acid to give off carbon dioxide.
If you added some washing soda crystals to vinegar,
what would you see happening?
Answers
A(C4H10O) reacts with phosphorus tribromide to give B(C4H9Br). B reacts
with sodium methoxide in THF at 0° C to give (S)-2-butyl methyl ether as the
major product.
Draw the structure of A.
Answers
Based on the given information, A is a molecule with the molecular formula C4H10O.
Chemical structure explained.Chemical structure refers to the arrangement of atoms, bonds, and other chemical groups in a molecule. It describes the spatial orientation of the atoms in a molecule and how they are connected to each other. The chemical structure of a molecule can be represented using a variety of different notations, including molecular formulas, Lewis structures, condensed structures, and three-dimensional models.
Based on the given information, A is a molecule with the molecular formula C4H10O. There are different isomers of C4H10O that could potentially be A, but one possibility is n-butanol (also known as 1-butanol). The structure of n-butanol is:
H H
\ /
C
/ \
C O
/ /
H H
Therefore, the structure of A (assuming it is n-butanol) is:
H H
\ /
C
/ \
C O
/ /
H H
Learn more about chemical structure below.
https://brainly.com/question/28353159
#SPJ1
The molecular mass of air, at standard pressure and temperature, is approximately 28.97 g/mol.
Calculate the mass of 3.33 moles of air.
First, complete the unit conversion using dimensional analysis:
A • B/C
A: answer: 3.33 mil air
B: answer: 28.87 g air
C: answer: 1 mol air
Answers
We are given:
Molar mass of air = 28.97 grams/mole
Amount of Air in question = 3.33 moles
Mass of 3.33 moles:
We know that:
mass = molar mass * number of moles
replacing the variables
mass = 28.97 \(\frac{grams}{mol}\) * 3.33 moles
[the 'mole' in the numerator and the denominator will cancel out]
mass = 28.97 * 33.3 grams
mass = 96.47 grams
In respiration,energy is released when oxygen and glucose react to make carbon dioxide and water, according to this equation:
CO₂ ₊ H₂O → C₆H₁₂O₆ ₊ O₂
Explain
why this reaction is an exothermic reaction, you should refer to bonds in your answer
Answers
Answer:
The reaction CO₂ + H₂O → C₆H₁₂O₆ + O₂ is exothermic because it releases energy. The energy is released because the bonds in the products (C₆H₁₂O₆ and O₂) are stronger than the bonds in the reactants (CO₂ and H₂O). Specifically, the reaction releases energy because the bonds between carbon and oxygen in CO₂ and the bonds between hydrogen and oxygen in H₂O are weaker than the bonds between carbon, hydrogen, and oxygen in C₆H₁₂O₆ and the bonds between oxygen atoms in O₂
The molar solubility of CoS is 5.0 × 10-22 mol L–1 in pure water. Calculate the Ksp for CoS.
Answers
Answer:
2.5 X 10^-43
Explanation:
CoS> Co + S
Co=X
S=X
X= 5.0 X 10^-22
Ksp= [X][X]
Ksp= [5.0 X 10^-22][5.0 X 10^-22]
Ksp= 2.5 X 10^-43
The Ksp for CoS is calculated as 2.5 × 10⁻⁶³ (mol/L)². This value represents the equilibrium constant for the dissociation of CoS in water and indicates the extent of its solubility in the solution.
The molar solubility of CoS is given as 5.0 × 10⁻²² mol/L. In the chemical equation for the dissolution of CoS in water, it dissociates into Co⁻²⁺ and S²⁻ ions.
The solubility product constant (Ksp) expression for CoS is written as:
Ksp = [Co²⁺] * [S²⁻]
Since CoS dissolves completely to form one Co²⁺ ion and one S²⁻ ion, we substitute the molar solubility values into the Ksp expression:
Ksp = (5.0 × 10⁻²² mol/L) * (5.0 × 10⁻²² mol/L)
Ksp = 2.5 × 10⁻⁴³ (mol/L)²
Therefore, the Ksp for CoS is calculated as 2.5 × 10⁻⁶³ (mol/L)². CoS has an extremely low solubility in water, resulting in an exceptionally small Ksp value.
To learn more about molar solubility here
https://brainly.com/question/28170449
#SPJ2
For the following reaction at equilibrium (400 °C), describe the effect on the equilibrium amount of Cl2(g) if additional O2(g) is added to the mixture at constant volume?

Answers
The addition of \(O_2(g)\) will shift the equilibrium towards the right side of the reaction and will consume \(Cl_2(g)\).
For the given reaction at equilibrium (400 °C), the effect on the equilibrium amount of \(Cl_2(g)\) if additional \(O_2(g)\)) is added to the mixture at constant volume can be determined by the Le Chatelier's principle.Le Chatelier's principle states that if a system in equilibrium is subjected to a stress, the system adjusts itself in such a way that it counteracts the stress and a new equilibrium is established.The given reaction is:\(Cl_2(g)\) + \(O_2(g)\) ⇌ 2ClO(g)When additional \(O_2\) is added to the mixture at constant volume, the concentration of O2(g) increases. According to Le Chatelier's principle, the system will adjust itself to counteract this increase in concentration by decreasing the concentration of \(O_2(g)\). This can be achieved by consuming \(O_2(g)\) to produce more ClO(g).The reaction shifts to the right to counteract the increase in concentration of \(O_2(g)\). As a result, the equilibrium amount of \(Cl_2(g)\) decreases, and the equilibrium amount of ClO(g) increases. Therefore, the addition of \(O_2(g)\) will shift the equilibrium towards the right side of the reaction and will consume \(Cl_2(g)\).Hence, the effect of adding \(O_2\) to the mixture will decrease the amount of \(Cl_2(g)\) at equilibrium while increasing the amount of ClO(g).Summary: If additional \(O_2\) is added to the mixture at constant volume, the concentration of \(O_2(g)\) increases. According to Le Chatelier's principle, the system will adjust itself to counteract this increase in concentration by consuming \(O_2(g)\) to produce more ClO(g). As a result, the equilibrium amount of \(Cl_2(g)\) decreases, and the equilibrium amount of ClO(g) increases. Therefore, the addition of \(O_2(g)\) will shift the equilibrium towards the right side of the reaction and will consume \(Cl_2(g)\).For more questions on equilibrium
https://brainly.com/question/29398344
#SPJ8
A flashbulb of volume 2.00 mL contains O2(g) at a pressure of 2.30 atm and a temperature of 20.0 °C. How many grams of O2(g) does the flashbulb contain?
Answers
Answer:
la bombilla de flash contiene 0,00550 gramos de O2
Explanation:
The distance of 24 km equals:
O 2.4x104 m
O 2.4x10-1 m
O 2.4x103 m
2.4x10-2 m
2.4x102 m
Answers
Answer:
2.4* 10⁴ m
Explanation:
1km = 1000m = 10³m
(1*24)km = 10³ * 24 m
=> 24km = 2.4 * 10⁴m
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
During winter, you can sometimes ice skate outdoors on a frozen lake. Why can’t you ice skate on a lake when it is not frozen?
Answers
Answer:
Generally the ice should be more than 4 inches thick to skate on it safely. However, the ice thickness is not always even and there can be thin spots, especially near springs or near river inlets or outlets. Most lakes and ponds don't completely freeze because the ice (and eventually snow) on the surface acts to insulate the water below. Our winters aren't long or cold enough to completely freeze most local water bodies. This process of lakes turning over is critically important to the life in the lake.
Explanation:
Why is it important o differentiate between human and animal blood from a crime scene? A) To determine whether an animal might have been injured. B) In order to accurately identify the source of any blood. In order to thoroughly and accurately recreate the crime scene. D To determine the possible source of any trace evidence such as hair or bite marks
Answers
Answer:
B) In order to accurately identify the source of any blood. In order to thoroughly and accurately recreate the crime scene.
i. Three different representations of glucose are
given below. Identify each type
of model.

Answers
Answer:
Ball and stick model is 3D and has the atoms depicted as different Coloured balls Conected to each other by "sticks"
fischer projection has the atoms on the side coming out of the plane, the atoms at the ends going behind (going away from you)
bond line notation Is the most common it does not show the C or H bonds but instead carbons are represented by the bends

The three structures of glucose represent three different models, they are ball and stick model, Fischer projection and bond line notation, respectively.
Glucose is a type of simple sugar (carbohydrate) that does not further hydrolyze to give monosaccharides.
Ball and stick model: The atoms are portrayed as different colored balls connected to each other by "sticks." Fischer projection: The atoms on the sides of the plane are coming out of the plane, while the atoms at the ends are moving behind.Bond line notation: It is the most frequent representation method used; it lacks C and H bonds but instead depicts carbons through bends.Therefore, the three glucose structures reflect three distinct models: the ball and stick model, the Fischer projection, and the bond line notation, respectively.
Learn more about Glucose here:
https://brainly.com/question/30548064
#SPJ5
19. Which of the following has 3 significant figures? 0.0730 O 300 4003
Answers
Answer:
0.0730
Explanation:
decimal 0s don't count except for ones at the end
Calculate the pH and percent ionization of a HC2H3O2 solution with a concentration of 0.500 M. (Ka = 1.8 x10-5)
Answers
Answer:
I can not see the file
Explanation:
List and explain each of the signs of a chemical change.
Answers
- precipitate(solid)forms: becomes cloudy or see sediment settle to the bottom
- release / absorption of energy: change in temperature or gives off light
- color change
Which of the following affects the potency of a drug?
The amount
Concentration
Number of exposures
Exposure method
Answers
Answer:
All of the listed factors can affect the potency of a drug.
Explanation:
All of the listed factors can affect the potency of a drug. Let's discuss each one:
The amount: The potency of a drug can be influenced by the dosage or amount administered. Generally, a higher amount of a drug can lead to a greater effect or potency. However, there may be optimal dosage ranges where the potency is maximized before plateauing or potentially causing adverse effects.
Concentration: The concentration of a drug refers to the amount of the drug present in a given volume or solution. A higher concentration of a drug can increase its potency since a greater quantity of the active substance is available to interact with the target receptors or sites.
Number of exposures: The number of times a person is exposed to a drug can also impact its potency. In some cases, repeated exposures can lead to an accumulation of the drug in the body, resulting in increased potency or stronger effects. However, this can also lead to tolerance, where the body becomes less responsive to the drug over time, requiring higher doses for the same effect.
Exposure method: The way a drug is administered or exposed to the body can affect its potency. Different routes of administration (e.g., oral, intravenous, inhalation, topical) can result in variations in the drug's absorption, distribution, and metabolism, which can influence its potency and onset of action.
It's important to note that potency is different from efficacy, which refers to the maximum therapeutic effect a drug can produce. Potency specifically relates to the amount of drug required to produce a particular effect.
6. You have 2.3 liters of gas at a pressure of 5.3 atm, and temperature of 45 °C. What will the temperature ofthe gas be if you decrease the volume of gas to 1.2L, and decrease the pressure to 2.5 atm ? 3 pts

Answers
We have a gas that we will assume behaves like an ideal gas. So we can apply the ideal gas law. The ideal gas law tells us:
\(PV=nRT\)Where,
P is the pressure of the gas
V is the volume of the gas
n is the moles of the gas
R is a constant
T is the temperature of the gas
We have two states of the gas. One initial and one final, for both states it is assumed that the moles remain constant. The conditions for each state are.
Initial state:
V1=2.3mL
P1=5.3atm
T1=45°C =318.15K
Final state:
V2=1.2L
P2=2.5atm
T2=?
For each state the ideal gas law will be:
\(\begin{gathered} \frac{P_1V_1}{T_1}=nR \\ \frac{P_2V_2}{T_2}=nR \end{gathered}\)Now, as the moles remain constant, the term nR will be constant and we can equate the two equations, we will then have that:
\(\frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2}\)Now, we clear T2 and replace the known data:
\(\begin{gathered} \frac{}{}T_2=\frac{P_2V_2}{P_1V_1}\times T_1 \\ T_2=\frac{2.5atm\times1.2L}{5.3atm\times2.3L}\times318.15K \\ T_2=78.30K \end{gathered}\)The temperature of the gas will be 78.3K
Which two bones make up the jaw?
Answers
Explanation:
The lower jaw (mandible), which also shapes the lower face and chin, supports the bottom row of teeth. The bone that moves when the mouth opens and shuts is this one. The upper jaw's (maxilla) function is to support the nose, hold the upper teeth, and shape the middle of the face.
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
The reaction in the diagram takes place in an ice calorimeter at 0°C.
What happens to the ice?
The temperature of the ice stays the same.
Some ice melts.
The ice gets colder.
You cannot tell from the information given.
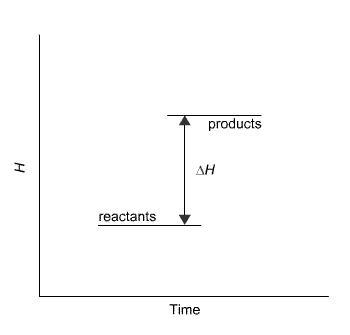
Answers
MgCl2 + 2 NaOH → 2 NaCl + Mg(OH)2
If you want to produce 11.00 moles of MgCl2, how many grams of NaOH are needed for the reaction to take place ?
Answers
To produce 11.00 moles of MgCl2, you would need 858.00 grams of NaOH.
To determine the amount of NaOH needed to produce 11.00 moles of MgCl2, we need to use stoichiometry and the balanced chemical equation:
\(MgCl_2 + 2 NaOH\) → \(2 NaCl + Mg(OH)_2\)
From the balanced equation, we can see that the mole ratio between \(MgCl_2\)and NaOH is 1:2.
Therefore, for every 1 mole of\(MgCl_2\), we need 2 moles of NaOH.
Given: Moles of \(MgCl_2\)= 11.00 moles
Using the mole ratio, we can calculate the moles of NaOH required:
moles of NaOH = 2 * moles of MgCl2
moles of NaOH = 2 * 11.00 moles
moles of NaOH = 22.00 moles
Now, we need to convert the moles of NaOH to grams using the molar mass of NaOH:
The molar mass of NaOH = 22.99 g/mol + 16.00 g/mol + 1.01 g/mol = 39.00 g/mol
grams of NaOH = moles of NaOH * molar mass of NaOH
grams of NaOH = 22.00 moles * 39.00 g/mol
grams of NaOH = 858.00 grams
Therefore, to produce 11.00 moles of \(MgCl_2\), you would need 858.00 grams of NaOH.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ8
What is the one thing that happens in every chemical change?
Answers
Answer:
Explanation:
Chemical reactions involve combining different substances. The chemical reaction produces a new substance with new and different physical and chemical properties. Matter is never destroyed or created in chemical reactions. The particles of one substance are rearranged to form a new substance.
Answer:
The chemical reaction produces a new substance with new and different physical and chemical properties. Matter is never destroyed or created in chemical reactions. The particles of one substance are rearranged to form a new substance. The same number of particles that exist before the reaction exist after the reaction.
Explanation: hope this helps