a sample of 63.6 grams of copper completely reacted with oxygen to form 71.6 grams of a copper oxide product. how many grams of oxygen must have reacted?
Answers
16.01 grams of oxygen must have reacted .
Calculation :
Reaction : 2Cu + O₂ -> 2CuO
Theoritical moles of Cu : O₂ = 2 : 1
Moles of Cu = mass/molar mass = 63.6/63.546 = 1.001 mol
Moles of O₂ = mass/molar mass = 71.6/32.0 = 2.238 mol
Experimental of Cu : O₂ = 1.001 : 2.238 = 0.4 : 1.0
Excess reactant is O₂
Limiting reactant is Cu
Moles of O₂ reacted = 0.5*moles of Cu
moles of O₂ reacted = 0.5 x 1.001 = 0.5004 mol
mass of O₂ reacted = moles x molar mass = 0.5004 x 32.00 = 16.01 g
mass of O₂ reacted is 16.01 g
A chemical reaction is the process of chemical transformation of one group of chemicals into another group. Classical chemical reactions involve changes that affect only the position of electrons in forming and breaking chemical bonds between atoms, with no changes in the nucleus (no changes in the elements present) and many If , it can be described by a chemical equation. Nuclear chemistry is a subfield of chemistry that deals with chemical reactions of unstable radioactive elements in which both electronic and nuclear changes can occur.
Learn more about reaction here : https://brainly.com/question/26283409
#SPJ4
Related Questions
The volume of a sample of nitrogen gas at a temperature of 291K and 1.0 x 105 Pascals was 3.5 x 10-2m3. Calculate the temperature at which the volume of the gas would be 2.8 x 10-2m3 at 1.0 x 105pascals.
Answers
Answer: \(232.8\ K\)
Explanation:
Given
The volume of Nitrogen gas is \(V_1=3.5\times 10^{-2}\ m^3\)
Pressure is \(P_1=10^5\ Pa\)
Temperature \(T_1=291\ K\)
When the pressure and temperature changes i.e.
\(P_2=10^5\ Pa\)
\(V_2=2.8\times 10^{-2}\ m^3\)
Temperature is given by
\(\Rightarrow \dfrac{P_1V_1}{T_1}=\dfrac{P_2V_2}{T_2}\\\\\Rightarrow T_2=\dfrac{P_2V_2}{P_1V_1}\times T_1\)
Insert values
\(\Rightarrow T_2=\dfrac{2.8\times 10^{-2}\times 10^5}{3.5\times 10^{-2}\times 10^5}\times 291\\\\\Rightarrow T_2=291\times 0.8\\\Rightarrow T_2=232.8\ K\)
Using the conversation factor of 12 eggs=1 doz, how many doz eggs are in 456 eggs
Answers
3. A man rode on his motorcycle for 162 miles. His average speed was 45 miles per hour. How long did his trip take?
Answers
Answer : 3.6 hours
what is hard water and soft water
Answers
Answer:
Hard water is water that has high mineral content, Soft water is free from dissolved salts of such metals as calcium, iron, or magnesium
Explanation:
Extra credit: Solve using dimensional analysis. A car averages 32. 5
mi/gallon. What is its mileage rate in m/dL?
Answers
The mileage rate of the car is approximately 52,383.55 meters per deciliter (m/dL) when given the average of 32.5 miles per gallon (mi/gallon).
To convert the mileage rate from miles per gallon (mi/gallon) to meters per deciliter (m/dL) using dimensional analysis, we need to apply conversion factors that relate the given units to the desired units.
Given:
Mileage rate = 32.5 mi/gallon
We can set up the dimensional analysis as follows, using the conversion factors:
32.5 mi/gallon * (1609.34 m/1 mi) * (1 gallon/3.78541 dL)
Let's break down the conversion factors used:
1 mi = 1609.34 m (conversion factor to convert miles to meters)
1 gallon = 3.78541 dL (conversion factor to convert gallons to deciliters)
Now, we can multiply the given mileage rate by the conversion factors:
32.5 mi/gallon * (1609.34 m/1 mi) * (1 gallon/3.78541 dL) = (32.5 * 1609.34) m/dL ≈ 52,383.55 m/dL
Therefore, the mileage rate of the car is approximately 52,383.55 meters per deciliter (m/dL) when given the average of 32.5 miles per gallon (mi/gallon).
learn more about mileage rate here
https://brainly.com/question/30167141
#SPJ11
the equivalence point of any acid-base titration can be determined visually from a titration curve by finding the place where
Answers
Answer:
where the slope of the titration curve is the greatest
The titration curve can be used to identify the equivalency point of the titration.The volume of titrant is where the titration curve has the steepest slope.
How do you find the equivalence point on a titration curve?
The equivalency point for acid-base titrations can be identified quite quickly.A simple pH meter is used to measure the pH of the solution being titrated after different amounts of titrant have been introduced to create a titration curve.The curve can then be read to determine the equivalency point. The equivalency point is identified using thermometric titrimetry, which gauges the rate at which a chemical reaction alters temperature.The inflection point in this instance denotes the threshold at which an exothermic or endothermic process is equivalent. The pH of a solution during a titration is represented graphically by a titration curve.The equivalence point in a strong acid-strong base titration is reached when the moles of acid and base are equal, and the pH is 7. The [H+] and [OH] concentrations must be equal at some point to be considered the equivalency point.Just a little bit beyond that is the endpoint, where the indicator color totally changes and the pH shifts from acidic to basic, or vice versa. The precise halfway point between the reactions of the titrant and the acid in the buffer solution is known as the half equivalence point.Because the pKa of the acid and the pH of the solution are equal at the half equivalence point, finding this point is not too difficult. A weak-acid/strong-base titration will have an equivalent point at a somewhat basic pH.The reason for this is that while the acid is not nearly as strong and does not completely dissociate to neutralize each equivalent of the base, the base is stronger and dissociates to a greater extent.To learn more about acid base titration refer
https://brainly.com/question/23687757
#SPJ2
Whenever Ayan returns from school he empties his water bottle in the potted plant instead
of throwing it in the sink. He always says his housemaid to use the water for watering plants
in the garden after mopping the house.
(a) Can you suggest any other use of the water left after mopping?
(b) What is the function of water in plants?
(c) What values of Ayan is shown here?
Answers
The ways to use of the water left after mopping is by using it to clean the gutters or drainage around your house or use it to water plants.
The function of water in plants is that it aid in photosynthesis.
The values of Ayan that is shown here is efficient use of water/ water sustainability
Why do we need water sustainability?Water is essential for healthy ecosystems, socioeconomic development, and human life, and it is at the heart of sustainable development.
It is essential for enhancing population health, welfare, and productivity as well as lowering the worldwide disease load. The ability to meet current water needs without compromising the capacity of future generations to do the same is known as sustainable water management.
Therefore, Photosynthesis, the process by which plants harness solar energy to make their own sustenance, requires water. Plants utilise hydrogen from water absorbed through their roots and carbon dioxide from the air during this process, releasing oxygen as a byproduct. Through pore-like stomas on the leaves, this exchange takes place.
Learn more about water sustainability from
https://brainly.com/question/1350753
#SPJ1
When 3.164 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 9.929 grams of CO2 and 4.065 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 28.05 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon.
Answers
The empirical formula of the hydrocarbon is CH2, and the molecular formula of the hydrocarbon is C2H4.
From the given data, we can obtain the empirical formula and molecular formula of hydrocarbon.
The given mass of hydrocarbon (CxHy) = 3.164 g
Given mass of CO2 formed = 9.929 g
Given mass of H2O formed = 4.065 g
First, we need to find out the number of carbon and hydrogen atoms in the given hydrocarbon.
To calculate the number of carbon atoms, we use the formula:
number of moles of CO2 formed = number of moles of carbon present in CxHy
Moles of CO2 = 9.929 g / 44.01 g/mol = 0.2258 mol
Thus, the number of moles of carbon present in CxHy is 0.2258 mol.
Also, each mole of carbon contains one mole of C atoms.
Hence, the number of carbon atoms in CxHy is also 0.2258 mol. Next, we calculate the number of hydrogen atoms using the formula: number of moles of H2O formed = number of moles of hydrogen present in CxHy
Moles of H2O = 4.065 g / 18.015 g/mol = 0.2258 mol
Thus, the number of moles of hydrogen present in CxHy is 0.2258 mol. Also, each mole of water contains two moles of hydrogen atoms. Hence, the number of hydrogen atoms in
CxHy is 2 × 0.2258 mol = 0.4516 mol
Empirical formula of the hydrocarbon = CH2
To find the molecular formula of the hydrocarbon, we need to know its molecular mass. The molar mass of the compound is given as 28.05 g/mol.
The empirical formula mass of CH2 is 14.03 g/mol.
Thus, the molecular formula of the hydrocarbon = (28.05 g/mol) / (14.03 g/mol) = 2 times the empirical formula
Therefore, the molecular formula of the hydrocarbon = C2H4
Learn more about empirical formula visit:
brainly.com/question/32125056
#SPJ11
Which of the following is the best method to make a racemic mixture of 2,3-dibromobutane (CH,CHBECHBECH,). A. photochemical bromination of 2-bromobutane B. addition of HBr to 3-bromo-2-butene (CH,CH-CBCH) C. addition of Br to cis-2-butene (cis-CH,CH-CHCH.) D. addition of Br, to trans-2-butene (trans-CH,CHCHCH)
Answers
The correct answer is D. addition of Br₂ to trans-2-butene (trans-CH₃CH=CHCH₃).
To form a racemic mixture, the starting compound should be an asymmetric molecule or a compound with an asymmetric center. In this case, 2,3-dibromobutane (CH₃CHBrCHBrCH₃) is an asymmetric molecule because it has two different bromine atoms attached to the central carbon.
The addition of bromine (Br₂) to trans-2-butene will result in the formation of 2,3-dibromobutane. Since trans-2-butene is an asymmetric starting material, the addition of bromine from both sides of the double bond will give rise to both possible enantiomers, leading to a racemic mixture.
Option A (photochemical bromination of 2-bromobutane) and option B (addition of HBr to 3-bromo-2-butene) do not involve an asymmetric starting material, so they won't result in a racemic mixture.
Option C (addition of Br₂ to cis-2-butene) also won't give a racemic mixture because cis-2-butene does not have an asymmetric carbon.
Therefore, the best method to make a racemic mixture of 2,3-dibromobutane is option D, the addtion of Br₂ to trans-2-butene.
Learn more about trans-2-butene here:
https://brainly.com/question/31498483
#SPJ11
What is the pOH of water?

Answers
Answer:
A. 7
(assuming the water is neutral)
3. If x electrons are needed to displace 108 g silver from a solution which contains Ag ions,
how many electrons are needed to displace 9 g of aluminium from a solution which contains
Al ions?
[Relative atomic mass Al, 27; Ag, 108]
A X
B 3x
C 4x
D 9x
Answers
Answer:
Choice A. \(x\) electrons would be required for displacing \(9\; \rm g\) of aluminum from a solution of \(\rm Al^{3+}\) ions.
Assumption: by "\(\rm Ag\) ions" the question meant \(\rm Ag^{+}\) with a charge of \(+1\) on each ion.
Explanation:
The question states that the relative atomic mass of \(\rm Ag\) is \(108\). In other words, each mole of
Therefore, that \(108\; \rm g\!\) of silver that were formed would contain \(1\; \rm mol\) of silver atoms.
Metallic silver would precipitate out of this \(\rm Ag^{+}\) solution only after these ions are turned into \(\rm Ag\) atoms.
One \(\rm Ag^{+}\) ion carries one unit of positive electrical charge. On the other hand, each \(e^{-}\) carries one unit of negative electrical charge.
Therefore, each \(\rm Ag^{+}\!\) ion will need to gain one electron to form a neutral \(\rm Ag\) atom.
\({\rm Ag^{+}}\; (aq) + e^{-} \to {\rm Ag}\; (s)\).
At least \(1\; \rm mol\) of electrons would be required to turn \(1\; \rm mol\!\) of \(\rm Ag^{+}\) ions into that \(1\; \rm mol\!\!\) of silver atoms (which have a mass of \(108\; \rm g\!\).)
Hence, \(x = 1\; \rm mol\).
Unlike \(\rm Ag^{+}\) ions, each aluminum ion \(\rm Al^{3+}\) carries three units of positive electrical charge. That is three times the amount of charge on one \(\rm Ag^{+}\!\) ion. Therefore, three electrons will be required to turn one \(\rm Al^{3+}\!\) ion to an \(\rm Al\) atom.
\({\rm Al^{3+}}\; (aq) + 3\, e^{-} \to {\rm Al}\; (s)\)
The question states that the relative atomic mass of \(\rm Al\) is \(27\). Therefore, each mole of \(\rm Al\!\) atoms would have a mass \(27\; \rm g\). There would be \(\displaystyle \frac{9\; \rm g}{27\; \rm g \cdot mol^{-1}} = \frac{1}{3} \; \rm mol\) of atoms in that \(9\; \rm g\) of \(\rm Al\!\!\).
It takes \(3\; \rm mol\) of electrons to turn one mole of \(\rm Al^{3+}\) ions to one mole of \(\rm Al\) atoms. Hence, \(\displaystyle \frac{1}{3}\times 3\; \rm mol = 1\; \rm mol\) of electrons would be required to produce that \(\displaystyle \frac{1}{3}\; \rm mol\) of \(\rm Al\!\) atoms (which has a mass of \(9\; \rm g\)) from \(\rm Al^{3+}\!\) ions.
That corresponds to the first choice, \(x\) electrons.
What are the products of the following equation?
Al2(SO4)3 + Ca3(PO4)2 ---> 2AlPO4 + 3CaSO4
Answers
136 grams of barium sulfate is used to make a 2.5 M solution. How many liters of water are needed?
Answers
Answer:
yay well done good job keep going
at a high altitude water boils at 95'c instead of 100'c as at sea level because
having a definite orderly arrangement of particle
the atmospheric pressure is less
its vapor pressure drops
Answers
At a high altitude, water boils at a lower temperature than at sea level because the atmospheric pressure is lower. Option B
What happens at high altitude?The atmospheric pressure decreases with increasing altitude because there is less air pressing against the water's surface.
When a liquid's vapor pressure reaches the same level as air pressure, boiling takes place. At sea level, the atmospheric pressure is 1 atm, hence for water to boil at 100°C, the vapor pressure must also be 1 atm. In contrast, the vapor pressure of water exceeds atmospheric pressure at lower temperatures at high elevations when the atmospheric pressure is lower.
Learn more about pressure:https://brainly.com/question/31940213
#SPJ1
To protect their crops during freezing temperatures, orange growers spray water onto the trees and allow it to freeze. In terms of energy lost and energy gained, explain why this practice protects the oranges from damage.
Answers
\(\huge{\textbf{\textsf{{\color{pink}{An}}{\red{sw}}{\orange{er}} {\color{yellow}{:}}}}}\)
When water freezes, it is an exothermic change. The oranges absorb this energy which is an endothermic change and do not freeze.
ThanksHope it helps.Which equation represents a spontaneous reaction?
A) Ca + Ba2+ to Ca2+ + Ba
B) Co + Zn2+ to Co2+ + Zn
C) Fe + Mg2+ to Fe2+ + Mg
D) Mn + Ni2+ to Mn2+ + Ni
Answers
Answer:Mn+Ni2+-->Mn2+ + Ni
Explanation:
Castle Learning said it
How do you determine if the reaction is spontaneous?
If ΔH is negative, and –TΔS positive, the reaction will be spontaneous at low temperatures (decreasing the magnitude of the entropy term).
What is the meaning of spontaneous reaction?
When both of these conditions are met, the reaction occurs naturally. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
Learn more about spontaneous reaction here : brainly.com/question/24376583
#SPJ2
consider a closed vessel initially containing 1 mol of pure ethyl acetate at 74°c and 100 kpa. imagine that pure ethanol is slowly added at constant temperature and pres¬sure until the vessel contains 1 mol ethyl acetate and 9 mol ethanol. describe the evo¬lution of phases and phase compositions during this process. comment on the practical feasibility of carrying out such a process. what sort of device would be required? how would the total system volume change during this process? at what composition would the system volume reach its maximum value?
Answers
By ideal gas approximation, the volume change is 230.2 liters.
We need to know about the ideal gas theory to solve this problem. The ideal gas is assumed that there is no interaction between particles in a gas. It can be determined by the equation
P . V = n . R . T
where P is pressure, V is volume, n is the number of moles gas, R is the ideal gas constant (8.31 J/mol.K) and T is temperature.
From the question above, we know that
n₁ = 1 mol
n₂ = 1 + 9 mol = 10 mol
P₁ = P₂ = 100 kPa = 100000 Pa
T₁ = 74⁰ C = 347 K
Find the initial volume
P₁ . V₁ = n₁ . R . T₁
100000 . V₁ = 1 . 8.31 . 347
V₁ = 0.0288 m³
When the initial and final temperature and pressure are the same, we can use the ratio of the number of moles and volume as
V₁ / n₁ = V₂ / n₂
0.0288 / 1 = V₂ / 9
V₂ = 0.25952 m³
V₂ = 259.52 liters
The volume change is
ΔV = V₂ - V₁
ΔV = 259.52 - 28.8
ΔV = 230.2 liters
Find more on ideal gas at: https://brainly.com/question/25290815
#SPJ4
What role does the cirrocumulus cloud play in the greenhouse effect?
(Science)
Answers
While cirrocumulus clouds may have some minor impact on the Earth's energy balance by reflecting sunlight, their role in the greenhouse effect is negligible compared to the greenhouse gases themselves.
Cirrocumulus clouds do not directly play a significant role in the greenhouse effect. The greenhouse effect refers to the process by which certain gases in the Earth's atmosphere trap heat from the sun, leading to an increase in the planet's temperature. The primary greenhouse gases responsible for this effect are carbon dioxide, methane, and water vapor.Cirrocumulus clouds, also known as high-level clouds, are composed of ice crystals and form at altitudes above 20,000 feet. They are thin, white, and often appear as small, rounded puffs or ripples in the sky. While these clouds can reflect some sunlight back into space, their overall impact on the greenhouse effect is minimal.In contrast, low-level clouds such as stratus or cumulus clouds can have a more significant influence on the greenhouse effect. These clouds have a higher potential to reflect incoming solar radiation and cool the Earth's surface, thus partially counteracting the warming effect caused by greenhouse gases.
for such more questions on greenhouse
https://brainly.com/question/14507701
#SPJ8
We will make about 350 mL of approx. 0.2 M NaOH (aq) solution by diluting 6 M NaOH (aq). Calculate the approximate volume of 6 M NaOH you need to make the diluted solution.
Answers
To make a 0.2 M NaOH (aq) solution, we will need to dilute 6 M NaOH (aq). we need approximately 11.67 mL of 6 M NaOH to make the diluted solution.
To determine the volume of 6 M NaOH required for the dilution, we can use the formula C1V1 = C2V2, where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume. In this case, we know the final concentration (0.2 M) and the final volume (350 mL). Therefore, we can rearrange the equation to solve for V1, the initial volume of 6 M NaOH needed for the dilution.
0.2 M * 350 mL = 6 M * V1
V1 = (0.2 M * 350 mL) / 6 M
V1 = 11.67 mL
Therefore, we need approximately 11.67 mL of 6 M NaOH to make the diluted solution.
To know more about diluted solution visit:
https://brainly.com/question/1416865
#SPJ11
a sample of a material has 20002000 radioactive particles in it today. your grandfather measured 40004000 radioactive particles in it 6060 years ago. how many radioactive particles will the sample have 60 years60 years from today?
Answers
The remaining mass of radioactive elements with 60 years of half-life is 1000 particles.
We need to know about the half-life of the radioactive elements to solve this problem. The radioactive element will decay over time and follow the equation
N = No(1/2)^(t/t'')
where N is the final quantity, No is the initial quantity, λ is the decaying constant, t is time and t'' is the half-life of a radioactive element.
From the question above, we know that
t'' = 60 years
No = 2000 particles
t = 60 years
Calculate the remaining mass
N = No(1/2)^(t/t'')
N = 2000 x (1 / 2)^(60 / 60)
N = 1000 particles
Find more on half-life at: https://brainly.com/question/25750315
#SPJ4
Which statement best describes what makes a base weak? A base is weak when it forms few ions in water. A base is weak when only a little of it is dissolved in water. A base is weak when its concentration is high. A base is weak when it totally forms ions in water.
Answers
Answer:
A base is weak when only a little of it is dissolved in water
Explanation:
Answer: B.) A base is weak when only a little of it is dissolved in water
Hope this helps :)
How many molecules are in 17 amu of ammonia, NH3?
A) 1.0 × 10²⁵
B) 2.0 × 10²⁴
C) 3.0 × 10²⁴
D) 6.0 × 10²³
Answers
6.0 × 10²³ are the molecules in 17 amu of ammonia, NH\(_3\). Therefore, the correct option is option D among all the given options.
What is molecule?The smallest recognizable unit in which a pure material may be split while retaining its composition and chemical characteristics is a molecule, which is a collection of two or more atoms.
Until portions made up of single molecules are reached, the split of a sample of a substance into increasingly smaller parts does not result in a change in either its makeup or its chemical characteristics.
molar mass of 1 mole of ammonia = 17 amu
1 mole of any substance contains 6.0 × 10²³
6.0 × 10²³ are the molecules in 17 amu of ammonia, NH\(_3\).
Therefore, the correct option is option D among all the given options.
To learn more about molecule, here:
https://brainly.com/question/29254782
#SPJ6
3.50 mol of CO2(g) is placed in a 7.00 L container. The following reaction occurs until equilibrium is established. Calculate the equilibrium concentrations of each species.
2CO2(g)⇌2CO(g)+O2(g) Kc=3.2×10−8
[CO2] = ? M
[CO] = ? M
[O2] = ? M
Answers
To solve this problem, we need to set up an ICE (Initial, Change, Equilibrium) table and use the given equilibrium constant (Kc) to determine the equilibrium concentrations. Let's start with the initial concentrations:
[CO2] = 3.50 mol / 7.00 L = 0.50 M (since the volume is given as 7.00 L)
[CO] = 0 M (since no CO is initially present)
[O2] = 0 M (since no O2 is initially present)
Next, let's assume that x mol/L of CO and O2 are formed at equilibrium. Since the stoichiometry of the reaction is 2:2:1 (2 CO2 : 2 CO : 1 O2), the change in concentration of CO and O2 will be equal to x mol/L.
Now, we can fill in the equilibrium concentrations in the ICE table:
| 2CO2(g) ⇌ 2CO(g) + O2(g)
--------------------------------------
Initial | 0.50 | 0 | 0
Change | -2x | +2x | +x
Equilibrium| 0.50 - 2x | 2x | x
According to the equilibrium constant expression (Kc), we have:
Kc = [CO]^2 * [O2] / [CO2]^2
Plugging in the equilibrium concentrations:
Kc = (2x)^2 * (x) / (0.50 - 2x)^2
Given that Kc = 3.2 × 10^(-8), we can set up the equation:
3.2 × 10^(-8) = (2x)^2 * (x) / (0.50 - 2x)^2
Solving this equation will give us the value of x, which represents the equilibrium concentration of O2. Once we have the value of x, we can calculate the equilibrium concentrations of CO and CO2 using the equilibrium expressions:
[CO] = 2x
[CO2] = 0.50 - 2x
Please note that solving the equation may involve using numerical methods, such as the quadratic formula or an equation solver.
To know more about Equilibrium refer here
https://brainly.com/question/11330806#
#SPJ11
a normal penny has a mass of about 2.5g. if we assume the penny to be pure copper (which means the penny is very old since newer pennies are a mixture of copper and zinc), how many atoms of copper do 9 pennies contain?
Answers
9 pennies contain approximately \(2.13 x 10^23\) atoms of copper.
To solve this problem, we need to use the following steps:
Determine the molar mass of copper.
Convert the mass of 9 pennies from grams to moles.
Use Avogadro's number to calculate the number of atoms of copper.
Step 1: The molar mass of copper (Cu) is approximately 63.55 g/mol.
Step 2: The mass of 9 pennies is:
9 pennies x 2.5 g/penny = 22.5 g
Converting this mass to moles, we get:
22.5 g / 63.55 g/mol = 0.354 moles
Step 3: Using Avogadro's number (\(6.022 x 10^23 atoms/mol)\), we can calculate the number of atoms of copper:
Therefore, 9 pennies contain approximately\(2.13 x 10^23 a\)toms of copper.
Learn more about molar mass
https://brainly.com/question/22997914
#SPJ4
Nova likes it when her days are unpredictable and filled with problems to solve. She thrives on solving puzzles and then testing out the different solutions in search of the "right fit." How would you describe One's personality
Answers
Nova's personality can be described as adventurous, problem-solving oriented, and driven by the pursuit of optimal solutions.
Nova's preference for unpredictable days and her enjoyment of solving puzzles suggests that she has an adventurous and curious nature. She thrives on the excitement and challenges that come with unexpected situations, finding them stimulating rather than overwhelming. Nova's ability to solve problems indicates a strong analytical and logical mindset. She enjoys the process of dissecting complex issues, identifying potential solutions, and engaging in trial and error to find the best fit.
This demonstrates her determination, perseverance, and willingness to explore different paths to achieve her goals. Nova's inclination towards testing out various solutions implies that she values experimentation and creativity, embracing the opportunity to think outside the box and discover innovative approaches. Her personality traits align with those of a problem-solver who actively seeks intellectual stimulation and embraces the satisfaction of finding optimal solutions to the challenges she encounters.
Learn more about logical mindset here-
https://brainly.com/question/31271234
#SPJ11
What is the mass in grams 52.9 moles of Xenon?
Answers
To find the mass in grams of 52.9 moles of Xenon, multiply the number of moles (52.9) by the molar mass of Xenon. The molar mass of Xenon is 131.293 g/mol. So, 52.9 moles of Xenon would have a mass of:
52.9 moles * 131.293 g/mol = 6,938.72 grams
Please help
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
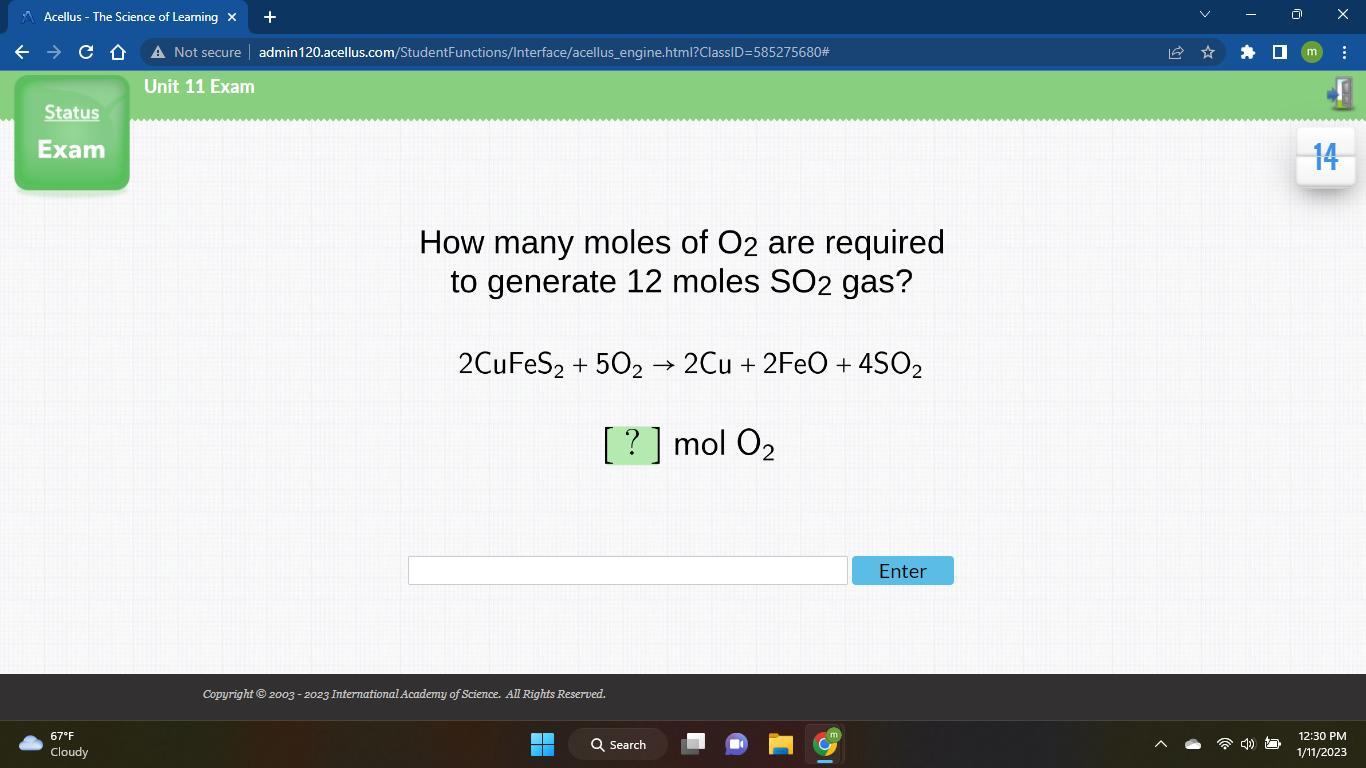
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
three difference between radicle and plumule
Answers
Answer:
1)RADICLE ->It is the embryonic root of the plant.
PLUMULE ->It is the embryonic shoot of the plant.
2)RADICLE ->It grows downwards into the soil.
PLUMULE ->It grows upwards into the air.
3)RADICLE ->Radicle is the first part of the seedling.
PLUMULE -> Plumule grows after the radicle.
4)Radicles are negatively phototropic. Plumule is positively phototropic.
i am glad i helped
what is the percent composition of nitrogen in N2S2
Answers
Answer:
composition of N₂S₂, you divide the total mass of each atom by the molecular mass and multiply by 100 %. Mass of 2 N atoms = 2 N atoms × 14.01u1N atom = 28.02 u.
A camper walked from point A to point B taking the path shown by the dotted line. What is the approximate distance the camper walked? a. 2.0 miles downhill b. 30 miles downhill c. 2.0 miles uphill d. 30 miles uphill
Answers
a. 2.0 miles downhill
This is because the path taken by the camper appears to go primarily downhill and is relatively short in distance. However, the actual distance may be more or less than 2.0 miles depending on the scale of the diagram.