Why is ΔSvap of a substance always larger than ΔSfus ?
Answers
Latent heat of fusion (ΔSfus) is basically the amount of energy required to melt one gram of a solid without changing its temperature.
Latent heat of vaporisation (ΔSvap) is basically the amount of energy required to vaporise (boil) one gram of a liquid without changing the pressure.
The strong intermolecular forces between liquid molecules are broken during vaporisation, converting the liquid to vapour. Thus, more energy is required to break the intermolecular attraction of liquid molecules than to simply weaken the bonds in solid to convert it to liquid (during fusion). As a result, a substance's latent heat of vaporisation is always greater than its latent heat of fusion.
Find more on latent heat of fusion and vaporisation at : https://brainly.com/question/17086277
#SPJ4
Related Questions
Give 3 physical properties common to most metals
Answers
Answer:
What are you a 6 yo? Anyways, the most common properties are, They are good conductors of electricity, They are lustrous and They are sonorous.
How to draw table for this type of question?

Answers
If you draw the table of the Hess law, you can use that table to obtain the enthalpy of reaction
How do you draw the table of the Hess law?A table called the "Hess's law table" can be created to depict how Hess's law is used. The reactants, intermediates, products, and related enthalpy changes (H) of each reaction that takes place during a chemical reaction are listed in the table.
Hess's law indicates that you can add the enthalpy changes of the separate reactions to get the total reaction's enthalpy change (H). By eliminating common species between neighboring reactions in the table, the overall reaction is achieved.
Learn more about Hess law:https://brainly.com/question/10504932
#SPJ1
1. How many grams of mercury occupy 225 mL?
Answers
• Answer:
m = 3,045.15 g mercur
• Explanation:
Hi there !
density formula
d = m/V =>
=> m = d×V
d(mercury) = 13.534 g/cm3
1ml = 1cm3
V = 225ml = 225cm3
replace
m = 13.534g/cm3×225cm3
m = 3,045.15 g mercur
Good luck !
It is known that oxygen contains 1 percent of the air. If 50 liters of wind, how much oxygen is needed? *
Answers
Answer:
25 PRECENT
Explanation:
assume that coal can be represented by the chemical formula c135h96o9ns . if 2.00 tons of coal is burned, what mass of nitrogen in the form of nox gases, is produced by this combustion?
Answers
If 2.00 tons of coal is burned, then the mass of nitrogen in the form of nox gases, is produced by this combustion is 400134 g of nitrogen.
The chemical reaction equation can be written as
C135H96O9NS + 156O2 ------- 135CO2 + 48H2O + NO + SO2
Mass of 2.0 tons of coal in gram = 1.814 × 10^(6) g
Molar mass of C135H96O9NS can be calculated as = 135(12) + 96(1) + 14 + 32 = 1904 moles of coal
Now, number of moles of coal burned = 1.814 × 10^(6) g / (1906 g/mol) = 952.7 moles of coal
If 1 mole of coal produced 1 mole of NO
Total 952.7 moles of coal produced 952.7 moles of NO
Mass of NO can be calculated as
Mass of NO produced = 952.7 × 30 = 28581 g
1 mole of NO = 30 g of NO
Thus, 14 g of nitrogen is contained in 30 g of NO
Let xg of nitrogen is contained in 28581 g of NO
Then,
x = 14 × 28581 = 400134 g of nitrogen.
If 2.00 tons of coal is burned, then the mass of nitrogen in the form of nox gases, is produced by this combustion is 400134 g of nitrogen.
learn more about combustion:
https://brainly.com/question/14335621
#SPJ4
Explain why a flash of green light made flubber so energetic
Answers
Answer:
The flash of green light may give off some type of energy that only emits from green light which probably energizes the Flubber
Explanation:
A flubber is a rubber like polymer that absorbs and amplifies energy. A flubber absorbs energy from a light source and electrons present in it are excited in order to emit light of higher wavelength. The green light absorbed by the flubber to emit longer wavelength of light emitting more energy.
Science students are studying specific heat capacity and trying to make decisions about what materials would be good insulators and conductors. Student groups setup this experiment:
Materials/group:
thermometer
Stopwatch
4 empty soda cans to be filled:
One can filled 1/2 way with water
One can filled 1/2 way with sand
One canfilled 1/2 way with iron filings
One empty can
Tub of ice water
Procedures:
Record the initial temperatures inside each can.
Place the four cans into the ice water bath, leaving them for 5 minutes.
Remove the cans and record the final temperatures of each can.
Question 6 (1 point)
Consider the data table as well as the experimental design. Which of the four cans should have the greatest change in temperature after five minutes?
Question 6 options:
iron filings
water
sand
air
Question 7 (1 point)
Once the activity had been completed, the instructor asked the students to design an insulated container they could use to keep 100mL of water hot. The only stipulation was that the insulator had to be a solid. The team that kept the water hot, at least 85° C, for the longest amount of time, would win a prize.
If you were participating, what would you use as your insulator?
Question 7 options:
sand
water
aluminum
cork
Answers
Air would be the most likely candidate for the can with the greatest change in temperature
What is the heat capacity?Recall that the change in the heat capacity would be related to the heat capacity.
Since it has a relatively low specific heat capacity compared to the other options listed (iron filings, water, and sand). However, it's important to note that this is a simplified explanation and there may be other factors at play depending on the specific conditions of the experiment.
Learn more about heat capacity:https://brainly.com/question/28302900
#SPJ1
 if your commute is 20 miles and you drive an average speed of 60 km/h how many minutes will it take you to get to work
Answers
Answer:
33 minutes
Explanation:
60 km/h = 37.28 mi/h
20/37.28 = 0.5364 h
0.5364 * 60 min = 32.2 minutes
How many elements of unsaturation do molecules with a molecular formula of c6h6cl6 have?
Answers
Molecules with the molecular formula C6H6Cl6 have one element of unsaturation. This means that the compound contains one double bond, one ring, or a combination of both. The molecular formula C6H6Cl6 represents a compound with six carbon atoms, six hydrogen atoms, and six chlorine atoms.
The degree of unsaturation is a measure of the presence of multiple bonds or rings in a compound. It can be calculated using the formula: Degree of unsaturation = (2C + 2 - H - X + N)/2
Where C is the number of carbon atoms, H is the number of hydrogen atoms, X is the number of halogen atoms (in this case, chlorine atoms), and N is the number of nitrogen atoms.
In the case of C6H6Cl6, the formula becomes: Degree of unsaturation = (2(6) + 2 - 6 - 6 + 0)/2
Degree of unsaturation = (12 + 2 - 6 - 6)/2
Degree of unsaturation = 2/2
Degree of unsaturation = 1
To know more about molecule visit:
brainly.com/question/31131977
#SPJ11
a contact burn occurs when a child's skin comes into contact with a flame or a hot solid object.
Answers
A contact burn occurs when a child's skin comes into contact with a flame or a hot solid object, such as a stove or curling iron.
This type of burn can result in tissue damage and requires proper care to prevent infection and promote healing. A contact burn is a type of burn that occurs when a child's skin comes into contact with a flame or a hot solid object. This can happen when a child touches a hot stove, grill, or even a candle flame. Contact burns can range from mild to severe depending on the temperature and duration of contact. It is important to immediately cool the burned area with cool water for at least 10-15 minutes and seek medical attention if necessary. Prevention is key in avoiding contact burns, so make sure to keep hot objects out of reach of children and teach them about the dangers of touching hot surfaces.
More on contact burn: https://brainly.com/question/28918945
#SPJ11
List all soluble salt
Answers
Answer:
Calcium, potassium, aluminum, chlorine, bromine
Explanation:
I do not know all of them, but here are some.
A 1.044 g sample contains only vitamin C (C6H8O6) and sucralose (C12H19Cl3O8). When the sample is dissolved in water to a total volume of 33.0 mL, the osmotic pressure of the solution is 3.69 atm at 295 K. What is the mass percent of vitamin C and sucralose in the sample?
Answers
Answer:
Refer the attachment for answer..
hope it helps you..




is this reaction oxidation or reduction
CH2=CH2 + OsO4 yields to HOCH2CH2OH
Answers
Answer:
it's definitely a oxidation reaction because here the oxygen contained OH group present in yeild
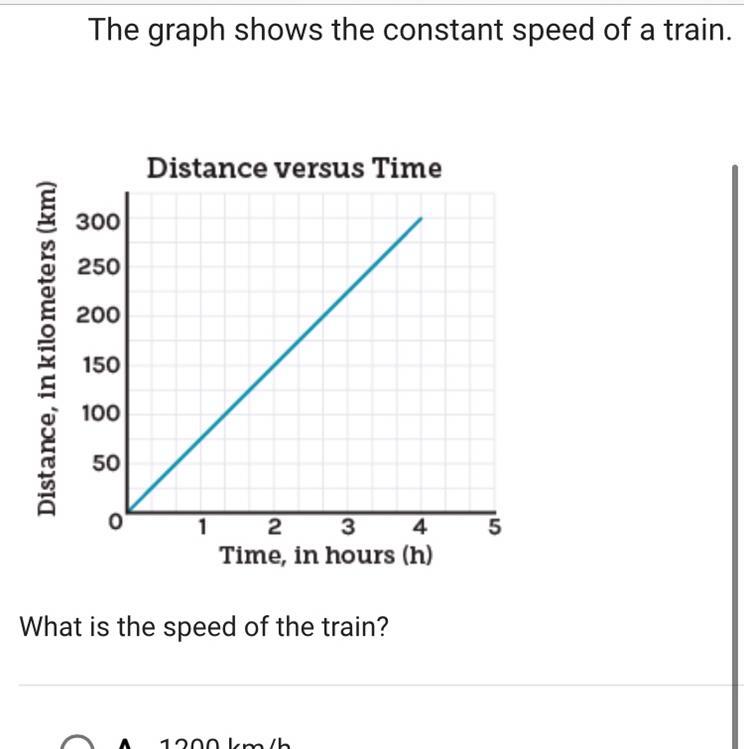
Please help me if your good with science:).
What is the speed of the train?
A.)1200km/h
B.)4 km/h
C.)75 km/h
D.)300 km/h
Answers
Identify the parts of a wave using the illustration and the function below.

Answers
Answer: 1. Crest 2. Trough 3. Wave Length 4. Amplitude
Explanation:
A hot air balloonist puts 52000 L of air into their balloon at 500 Celsius and 975 atm. When they heat
the air to 750 celsius what is the final volume (in cm^3) in the balloon?
Answers
Answer: The final volume in the balloon is \(68.818cm^3\)
Explanation:
Charles' Law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles of gas.
Mathematically,
\(\text{Volume}\propto \text{Temperature}\)
Or,
\(\frac{V_1}{T_1}=\frac{V_2}{T_2}\) (At constant pressure and number of moles)
\(V_1\) = initial volume = 52000 L
\(V_2\) = final volume = ?
\(T_1\) = initial temperature = \(500^0C=(500+273)K=773 K\)
\(T_2\) = final temperature = \(750^0C=(750+273)K=1023 K\)
\(\frac{52000}{773}=\frac{V_2}{1023}\)
\(V_2=68818L=68.818ml=68.818cm^3\) \((1L=1000ml=1000cm^3)\)
Thus final volume in the balloon is \(68.818cm^3\)
Why
would scientists synthesize natural resources
Answers
Answer:
so that the can keep reusing it and reusing it instead of wasting materials
Explanation:
you very welcome have a good day
What statement describes the cause for sibling rivalry between both brothers? The older son shared his fears about providing financial support for the family after graduating. The sons challenged each other by competing for the highest grade point average that year. The younger son expressed feelings about his parents showing favoritism to his older sibling. The sons disagreed over the handling of family tasks once the younger son heads off to college
Answers
Answer:
c
Explanation:
you took the test on edge
The statement describes the cause for sibling rivalry between both brothers is The younger son expressed feelings about his parents showing favoritism to his older sibling. Hence , Option (C) is correct.
What is Sibling rivalry ?Sibling rivalry is the jealousy, competition and fighting between brothers and sisters.
It is a concern for almost all parents of two or more kids.
Problems often start right after the birth of the second child.
Sibling rivalry usually continues throughout childhood and can be very frustrating and stressful to parents.
Therefore, The statement describes the cause for sibling rivalry between both brothers is The younger son expressed feelings about his parents showing favoritism to his older sibling. Hence , Option (C) is correct.
Learn more about Sibling rivalry here ;
https://brainly.com/question/11790181
#SPJ2
calculate the wavelength of one 19 f 1 ion that is moving with a speed of 4.255 x 10 5 m/s. a. 4.936 x 10 -17 m b. 4.936 x 10 -14 m c. 1.484 x 10 -29 m d. 1.484 x 10 -26 m e. 4.936 x 10 -20 m
Answers
The wavelength of one 19 f 1 ion that is moving with a speed of 4.936 x 10 -14 m
λ = h/p = h/mv = 6.625 × \(10^{-34}\) / 3.15 × \(10^{-26}\) = 4.936 x 10 -14 m
The length that a periodic wave travels across in order to replicate its shape is known as its wavelength. It is the distance between two successive corresponding wave points of the same phase, such as two neighboring crests, troughs, or zero crossings, and it is a feature of b0th traveling waves and standing waves and spatial wave patterns. The spatial frequency is defined as the wavelength's inverse. The Greek letter lambda, or "wavelength," is frequently used to represent it. Additionally, sinusoid wave envelopes, modulated waves, and waves created by the interference of several sinusoids are also commonly referred to as having a wavelength.
Wavelength and frequency are inversely related, assuming a sinusoidal wave traveling at a constant speed: in waves.
Learn more about wavelength here:
https://brainly.com/question/12924624
#SPJ4
Calculate the amount of heat (in kJ) that must be absorbed to convert 108 g of ice at 0oC to water at 70oC.
Answers
"The amount of heat that must be absorbed to convert 108 g of ice at 0°C to water at 70°C is approximately 68.12 kJ."
To calculate the amount of heat required to convert ice at 0°C to water at 70°C, we need to consider two steps:
Heat required to raise the temperature of ice from 0°C to its melting point (0°C).
Heat required to melt the ice at its melting point (0°C) and raise the temperature of water from 0°C to 70°C.
Let's calculate the heat for each step:
Step 1: Heating ice from 0°C to its melting point (0°C)
The specific heat capacity of ice is 2.09 J/g°C.
Heat = mass × specific heat capacity × change in temperature
Heat = 108 g × 2.09 J/g°C × (0°C - 0°C)
Heat = 0 kJ (No heat is absorbed as there is no change in temperature)
Step 2: Melting ice and heating water from 0°C to 70°C
The enthalpy of fusion (heat of fusion) for ice is 334 J/g, which represents the amount of heat required to melt ice at 0°C.
Heat for melting ice = mass × enthalpy of fusion
Heat for melting ice = 108 g × 334 J/g
Heat for melting ice = 36,072 J
Next, we need to calculate the heat required to raise the temperature of the water from 0°C to 70°C.
The specific heat capacity of water is 4.18 J/g°C.
Heat = mass × specific heat capacity × change in temperature
Heat = 108 g × 4.18 J/g°C × (70°C - 0°C)
Heat = 32,043.6 J = 32.04 kJ
Now, we can sum up the heat required for both steps:
Total heat = Heat for melting ice + Heat for raising water temperature
Total heat = 36,072 J + 32,043.6 J
Total heat = 68,115.6 J = 68.12 kJ
Therefore, the amount of heat that must be absorbed to convert 108 g of ice at 0°C to water at 70°C is approximately 68.12 kJ.
To know more about heat visit:
https://brainly.com/question/934320
#SPJ11
What volume of 0.100M NaOH is required to completely neutralize 15.00mL of 0.100M H3PO4?Select one:a.45 mLb.15 mLc.60 mLd.30 mL
Answers
45 ml of 0.100M NaOH is required to completely neutralize 15.00mL of 0.100M H₃PO₄.
A neutralization reaction is when an acid and a base react to shape water and salt and includes the aggregate of H+ ions and OH- ions to generate water. The neutralization of a robust acid and strong base has a pH same to 7.
Neutralization is a chemical reaction in which acid and a base react quantitatively with each different. In a response in water, neutralization results in there being no extra hydrogen or hydroxide ions present within the solution.
Given;
M₁ = 0.100M NaOH
valance factor n₁ = 1
V₁ = ?
M₂ = of 0.100M H3PO4
valance factor n₂ = 3
V₂ = 15.00mL
M₁n₁V₁ = M₂n₂V₂
V₁ = M₂n₂V₂/M₁n₁
= 0.1 × 3 × 15 / 0.1 × 1
= 45 ml
Learn more about neutralization here:-https://brainly.com/question/23008798
#SPJ9
If the concentration of H3O+ in an aqueous solution is 7.6 × 10-9 M, the concentration of OH- is ________.A) 7.6 × 10-23 MB) 1.3 × 10+8 MC) 6.4 × 10-5 MD) 1.3 × 10-6 ME) 7.6 × 10-9 M
Answers
The concentration of OH- in the aqueous solution is approximately 1.3 × 10-6 M, which corresponds to option D.
To find the concentration of OH- in an aqueous solution when the concentration of H3O+ is 7.6 × 10-9 M, we can use the ion product constant for water (Kw):
Kw = [H3O+][OH-]
Kw is a constant value at 25°C, equal to 1.0 × 10-14.
Given [H3O+] = 7.6 × 10-9 M, we can solve for [OH-]:
1.0 × 10-14 = (7.6 × 10-9)[OH-]
To find [OH-], divide both sides of the equation by the [H3O+] value:
[OH-] = (1.0 × 10-14) / (7.6 × 10-9)
[OH-] ≈ 1.3 × 10-6 M
So, the concentration of OH- in the aqueous solution is approximately 1.3 × 10-6 M, which corresponds to option D.
Learn more about concentration
brainly.com/question/3045247
#SPJ11
What is meant by the phrase "a consistent method of measurement"?
O A. The same person performs all measurements.
B. Variables are measured using the same method each time.
OC. All measurements are in non-Sl units.
O D. You only take one measurement.

Answers
what type of bond is this combination most likely to form?
Answers
Sodium is a group 1 element with atomic number 1. It has 11 electrons. It is soft reactive metal. It has 1 valence electron.
Fluorine is a group 7 element, a hologen with 7 valence electron. It is a most reactive non metal.
When sodium react with fluorine, ionic bond is formed in the resulting compound sodium fluoride.
One sodium and fluorine each totaling 2 atoms are enough to make the bond.
As the bond is formed, both atoms have octet structure. That is they each have 8 electrons on their outermost shells.
The positive charge on sodium indicates that sodium had lost 1 electron to fluorine atom.
The negative charge on fluorine ion indicates that fluorine atom had gained 1 electron from sodium atom to form negative ion.
The name of the compound is sodium fluoride with formula NaF.
brainliest please <3
_____ is a volume in which no matter exists.
Answers
A vacuum is a volume in which no matter exists. It is essentially an empty space with no particles or atoms present. In the natural world, a perfect vacuum does not exist, but we can create near-vacuum conditions in a laboratory setting. In addition, vacuums can be created artificially using various techniques, such as pumping out all the air from a container or using high-powered vacuum pumps. In space, there are areas with very low densities of particles, which are often referred to as vacuum environments. However, even if these areas contain no matter, it still has properties such as pressure, temperature, and energy, which can be measured and manipulated. #SPJ11
what is the product after the chemical reaction

Answers
Answer: a. 2 C3H7OH + 9 O2 → 6 CO2(g) + 8 H2O(g)
b. C6H12O6 + 6 O2 → 6CO2(g) + 6 H2O(g)
Explanation:
Alcohols and sugars combust or when burned with oxygen forms carbon dioxide and water.
200 grams of C3H4 gas with 20% purity is burned with excess O2 gas. How many grams of H2O are
produced with 60% yield?
Answers
Answer:
Explanation:
approx 30%
Reaction
C₃H₄ + 4O₂ → 3CO₂ + 2H₂O
20% purity = 20% x 200 = 40 g
mole C₃H₄ = mass : molar mass = 40 : 40 g/mole = 1
mole H₂O from reaction coefficient (C₃H₄ limiting reactant) = 2/1 x 1 mole = 2 mole
mass H₂O = mole x molar mass = 2 x 18 g/mole = 36 g
for 60% yield, H₂O produced = 60% x 36 g = 21.6 g
How many milliliters of a 6.50 M stock solution would be required to produce 500 mL of a 0.25 M dilute solution? Ms:Md: Vs:Vd: Answer:
Answers
You can see that Ms and Vs correspond to the stock solution, whereas Md and Vd corresponds to the dilute solution. The word 'M' means concentration and 'V' means volume. We want to know what is the volume of the stock solution (Vs). So, we have to use the following formula:
\(C_1V_1=C_2V_2.\)Where C is concentration and V volume. We can write this formula in our terms, like this:
\(M_sV_s=M_dV_d.\)Let's solve for 'Vs':
\(V_s=\frac{M_dV_d}{M_s}\text{.}\)The given data is:
\(\begin{gathered} M_s=6.50\text{ M,} \\ M_d=0.25M,_{} \\ V_d=500\text{ mL.} \end{gathered}\)So, replacing these values in the last formula, we're going to obtain:
\(V_s=\frac{0.25\text{ M }\cdot\text{ 500 mL}}{0.25\text{ M}}=19.2307\text{ mL}\approx19.2\text{ mL.}\)The answer is that we have 19.2 milliliters (mL) in the stock solution.
Suppose that 75mL of 4. 25 M iron (III) chloride is combined with 81mL of 3. 50 M calcium chloride. What is the concentration of Cl- ion in the resulting solution?
Answers
The concentration of Cl- ion in the resulting solution is 6.12 M.
This can be calculated by finding the moles of Cl- ion in each solution, adding them together, and dividing by the total volume of the combined solutions. To find the moles of Cl- ion in the iron (III) chloride solution, we can use the formula: moles = concentration x volume. Thus, moles of Cl- ion = 4.25 M x 0.075 L = 0.31875 moles. Similarly, for the calcium chloride solution, we have moles of Cl- ion = 3.50 M x 0.081 L = 0.2835 moles.
Adding these two moles together gives a total of 0.60225 moles of Cl- ion in the combined solution. Dividing this by the total volume of the combined solutions (75 mL + 81 mL = 156 mL = 0.156 L) gives a concentration of 6.12 M. Therefore, the resulting solution has a Cl- ion concentration of 6.12 M, which is higher than the individual concentrations of the original solutions. This is because the two solutions have been mixed together, leading to an increase in the total number of Cl- ions in the resulting solution.
For more questions like Ions visit the link below:
https://brainly.com/question/15051468
#SPJ11
Ill mark brainleist please helppp

Answers
Answer:
rusting, cooking and burning wood