Answers
Answer
(2) Chemical energy is converted to electrical energy.
Explanation
A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. In a voltaic cell, the anode is an electrode where oxidation occurs and the cathode is an electrode where reduction occurs.
Therefore, the process that occurs in an operating voltaic cell is:
(2) Chemical energy is converted to electrical energy.
Related Questions
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
1
Which of the following is an example of a nonrenewable
resource?
A
B
C
D

Answers
Answer:
B not really sure tho :)
Explanation:
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
What happens to the molecule If energy is removed from a substance
Answers
Answer:
Removing energy (cooling) atoms and molecules decreases their motion, resulting in a decrease in temperature
uses of sodium chloride in daily life
Answers
Answer:
sodium chloride can be used as salt
extraction sodium metal by electrolysis
a common chemical in laboratory experiments
Answer:
sodium chloride can be used as preservatives,
in preserving foods.
How many people died from pollution in 2007
Answers
Answer:
428,715
Explanation:
World figures say that around 428,000 people died of pollution in 2007.
What important material is absorbed by your digestive system besides water
Answers
Answer:
The monosaccharides, amino acids, bile salts, vitamins, and other nutrients are absorbed by the cells of the intestinal lining
Explanation:
Answer:
The monosaccharides, amino acids, bile salts, vitamins, and other nutrients are absorbed by the cells of the intestinal lining.
Explanation:
Liquid nitrogen is kept at a temperature of -320 degrees. When liquid nitrogen is heated it quickly boils and turns into a gas. Which pair of pictures represent the change caused by adding heat to liquid nitrogen?
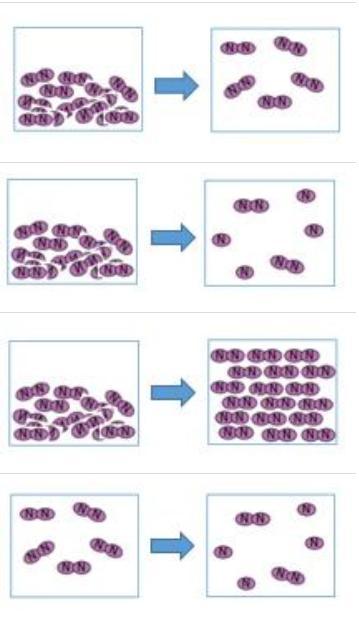
Answers
So when something is boiling it is moving faster meaning the molecules are more spread out and not condense like a solid
So the answer is the 2nd set
if an endothermic reaction begins at 26°C and decreases by 2°C per minute how long will it take to reach 0°C?
Answers
Answer:
13 minutes
Explanation:
For the endothermic reaction to reach 0°C, it will take 13 minutes.
Let us follow the process step by step;
Rate of decrease is 2°C per minute.
Start is 26°C
Time temperature
0 min 26°C
1 min 24°C
2 min 22°C
3 min 20°C
4 min 18°C
5 min 16°C
6 min 14°C
7 min 12°C
8 min 10°C
9 min 8°C
10 min 6°C
11 min 4°C
12 min 2°C
13 min 0°C
Starting with appropriate unlabeled organic compounds, show syntheses of each of the following:
Draw the reagents needed to produce C6H5—C≡C—T.
Answers
In first reaction alkyne is treated with base to remove proton later alkyne anion react with T20 to form tritium isotope labelled product.
What is reagent?
Reagent is a substance used to bring about a chemical reaction, or added to test if a reaction occurs. In a chemical reaction, one or more reactants are combined to form one or more products. A reagent is typically used to start, speed up, or determine the progress of a reaction. Common reagents include acids, bases, oxidizers, reducing agents, and salts. Reagents can also be used in qualitative or quantitative analysis to detect, measure, or separate chemicals in a sample.
For structure refer attached file
For more information about reagent please visit:
https://brainly.com/question/30024442
#SPJ4

After being treated with a base in the initial reaction to remove the proton, the alkyne anion then reacts with T20 to produce a product labeled with the tritium isotope.
A reagent in a chemical process is what?
In the field of chemical research, a "substance or compound that is given to a system in order to bring about a chemical reaction or is added to check whether a reaction is occurring or not" is referred to as a reagent. A similar response is utilized to validate the discovery of the presence of another drug.
A catalyst can speed up a certain chemical reaction, whereas a reagent is a material or mixture used in chemical analysis or other reactions.
See attached file for response.
To learn more about reagent use link below:
https://brainly.com/question/26905271
#SPJ4

For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M, and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant (Kc) for the given reaction is approximately 16.448. The value of Kc indicates the relative concentrations of reactants and products at equilibrium. In this case, a Kc greater than 1 suggests that the products (H2 and P4) are favored at equilibrium, indicating that the forward reaction is more favorable.
To determine the equilibrium constant (Kc) for the given reaction:
4PH3(g) ↔ 6H2(g) + P4(g)
We can write the equilibrium constant expression based on the stoichiometric coefficients:
Kc = ([H2]^6 * [P4]) / ([PH3]^4)
Substituting the given equilibrium concentrations:
[PH3] = 0.250 M
[H2] = 0.580 M
[P4] = 0.750 M
We can plug in these values into the equilibrium constant expression:
Kc = ([0.580]^6 * [0.750]) / ([0.250]^4)
Kc = (0.0860128 * 0.750) / (0.00390625)
Kc = 16.448
for more question on equilibrium
https://brainly.com/question/18849238
#SPJ8
convert 3.49g/cm³ to kg/m³
Answers
1 g/cm3 to kg/m3 = 1000 kg/m3
3.49 g/cm³= 3.49 x 1000
= 3490 kg/m³
Ans: 3490 kg/m³
Consider the reaction, CH4 (g) +202 (g) → CO₂ (g) + 2H₂O (1), AH= −890 kJ.
What will be the change in enthalpy when 3 moles of methane react in excess oxygen?
O-890 kJ
○ -2.67 × 10³ kJ
O +890 kJ
O +2.67 x 10³ kJ
Answers
The change in enthalpy when 3 moles of methane react in excess oxygen is -2.67 × 10³ kJ (Option B).
What is enthalpy?Enthalpy (H) is a thermodynamic property that describes the total heat content of a system at a constant pressure. It is a measure of the energy that is transferred as heat during a chemical reaction or physical change at constant pressure.
Enthalpy is defined mathematically as:
H = U + PV
where U is the internal energy of the system, P is the pressure, and V is the volume. Enthalpy is often measured in units of Joules (J) or kilojoules (kJ).
The given reaction is:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ΔH = -890 kJ
This equation shows that when one mole of methane reacts with two moles of oxygen, it produces one mole of carbon dioxide and two moles of water while releasing 890 kJ of energy.
To determine the change in enthalpy when 3 moles of methane react in excess oxygen, we need to first calculate the amount of heat released when one mole of methane reacts with excess oxygen.
From the balanced chemical equation, we can see that 1 mole of CH4 releases 890 kJ of energy, so the energy released by 3 moles of CH4 would be 3 times that value:
Energy released by 3 moles of CH4 = 3 × (-890 kJ/mol) = -2670 kJ
To know more about internal energy, visit:
https://brainly.com/question/14668303
#SPJ1
The change in enthalpy when 3 moles of methane react in excess oxygen is -2670 kJ.
What is Enthalpy?
Enthalpy is a thermodynamic property of a system that describes the heat content of the system at constant pressure. It is denoted by the symbol "H" and is expressed in units of joules (J) or kilojoules (kJ). Enthalpy is a state function, which means that it depends only on the initial and final states of the system, not on the path taken to reach those states.
The given reaction is: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l), ΔH = −890 kJ
This reaction is for one mole of methane. To find the change in enthalpy when 3 moles of methane react, we need to multiply the enthalpy change by 3:
ΔH = 3 × (-890 kJ/mol) = -2670 kJ
Therefore, the change in enthalpy when 3 moles of methane react in excess oxygen is -2670 kJ.
Learn more about Enthalpy from given link
https://brainly.com/question/14047927
#SPJ1
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
I need help on #10 please this isn’t graded thank you

Answers
The elements which can form anions among the following is O, S and Se.
What are anions?
Anions are the negatively charged ions which are formed by the balanced atoms when they gain one or more electrons to become negatively charged. The examples for anions include, Cl-, F-, O2-, SO4- etc. Usually the anions are formed by the non metals when they gain electrons.
The non metals like O, Se and S have a tendency to form anions because they are just 2 electrons away from completing the octet in the configuration. So, they easily accept 2 electrons to fill the octet and become stable forming anions with other atoms.
Therefore, the elements like O, S and Se form anions faster than the other elements in the following.
To learn more about the anions click on the given link https://brainly.com/question/14309645
#SPJ1
What is the order of reagents that were added to the test tube used to carry out the synthesis of dibenzalacetone?
Answers
Answer:
An experiment designed to synthesize dibenzlacetone would have reagents added in the following order:
Sodium hydroxide, NaOHEthanolAcetoneBenzaldehydeYellow precipitate would be re-crystallized from ethyl acetate.Explanation:
It is worth noting that the reaction is a type of mixed aldol condensation reaction, which uses sodium hydroxide as base.
I hope this is was a clear solution to the problem.
What color flame did lead nitrate produce?
yellow-red
blue
green
purple
Answers
Answer: it blue
Explanation:
1. Which statement is true about the relationship between chromosomes, genes and
traits?
A. Genes are found within traits, and their codes are used to make chromosomes.
B. Chromosomes are found within genes, and their codes are used to make traits.
C. Genes are found within chromosomes, and their codes are used to make traits.
D. One gene is found on every chromosome and they are used to make traits.
Answers
Answer:
the answer is C.Explanation:
Given the empirical formula C2H3O, what is the molecular formula that has a mass of 214.8g?
Answers
Given the empirical formula, the molecular formula that has a mass of 214.8g is C₁₀H₁₅O₅
Empirical & ,molecular formulaeFrom the question, we are to determine the molecular formula
From
Molecular formula = (Empirical formula)n
Where
\(n = \frac{Molecular\ formula\ mass}{Empirical\ formula\ mass}\)
From the given information,
Empirical formula is C₂H₃O
Therefore, Empirical formula mass = (12×2) + (1×3) + (16)
Empirical formula mass = 24 + 3 + 16
Empirical formula mass = 40 g
Then,
\(n = \frac{214.8}{40}\)
n = 5.37
n ≅ 5
Therefore,
Molecular formula = (C₂H₃O)₅
Molecular formula = C₁₀H₁₅O₅
Hence, given the empirical formula, the molecular formula that has a mass of 214.8g is C₁₀H₁₅O₅
Learn more on Empirical and molecular formulae here: https://brainly.com/question/1603500
Chemicals that control weeds are called
A. acaricides.
B. herbicides.
C. insecticides.
D. pesticides.
Answers
Answer:
The answer is D
Explanation:
because insecticides are used for insects but pesticides are used to kill off unwanted plants
Answer:
B. herbicides
Explanation:
it kills weeds.
Calculate the mass percent by volume of 330.1 g of glucose (C₆H₁₂O₆, MM = 180.2 g/mol) in 325 mL of solution.
Answers
Answer: The mass percent by volume is 101.6%
Explanation:
The solution concentration expressed in percent by volume means that the amount of solute present in 100 parts volume of solution.
It is represented in formula as :
mass percent by volume =\(\frac{\text {mass of solute}\times 100}{\text {Volume of solution in ml}}\%\)
Given : mass of glucose = 330.1 g
volume of solution = 325 ml
Thus mass percent by volume =\(\frac{330.1g\times 100}{325ml}=101.6\%\)
Thus the mass percent by volume is 101.6%
Electrochemical cells generate electricity from which of the following? Select all that apply.
electron transfer
flow of electrons
dissolving an ionic compound
redox reactions
Answers
By a redox reaction that involves the transfer of electrons, often through the dissolution of an ionic substance, electrochemical cells produce electricity from the flow of electrons.
What fuels the production of energy by electrochemical cells?In electrochemistry, redox or oxidation-reduction reactions, in which electrons travel from one element to another, can produce electricity. Redox processes involve the transfer of electrons from one substance to another.
In what element are electrochemical cells made?Batteries use a very significant class of oxidation and reduction reactions to produce useable electrical energy. Using solutions of respective sulphates, copper and zinc metals can be combined to create a straightforward electrochemical cell.
To know more about electrons visit:-
https://brainly.com/question/20513633
#SPJ1
(a) Calculate the pH of a buffer that is 0.12 M in lactic acid and 0.11 M in sodium lactate. (b) Calculate the pH of a buffer formed by mixing 85 mL of 0.13 M lactic acid with 95 mL of 0.15 M sodium lactate.
Answers
Answer:
fe9ufeohdwbkdwsdjvdwihdwkbfw
does anyone know the answer to this

Answers
What is the density of a piece of granite whose volume is 20 mL and mass is 53
grams?
3.05 g/mL
2.75 g/mL
4.0 g/mL
2.65 g/mL
Answers
2.65g/ml is the density of a piece of granite whose volume is 20 mL and mass is 53grams. Density is the mass of a specific material per unit volume.
What is density?Density is the mass of a specific material per unit volume. Density is defined as d = M/V, in which d represents density, M is weight, as well as V is volume. Density is generally expressed in grams every cubic centimetre. Water, for example, has a density of 1 gram per square centimeter, but Earth has a density of 5.51 kilograms per cubic centimetre.
Density is sometimes measured in kilos per cubic centimeter (in metre-kilogram-second or SI units). The density of air, for example, is 1.2 kilos per cubic metre.
density = mass / volume
=53/ 20
=2.65g/ml
Therefore, 2.65g/ml is the density of a piece of granite whose volume is 20 mL and mass is 53grams.
To learn more about density, here:
https://brainly.com/question/13434141
#SPJ1
Experiments were done on a certain pure substance X to determine some of its properties. There's a description of each experiment in the table below. In each case, decide whether the property measured was a chemical or physical property of X, if you can. If you don't have enough information to decide, choose can't decide in the third column.
Property P: A small sample of X is dissolved in water. Drops of another solution, containing dissolved sodium hydroxide, are slowly added, and a pH indicator is used to determine when the sodium hydroxide has completely reacted with X. From the amount of sodium hydroxide needed, the value of P may be calculated.
Property D: A sample of X is carefully weighed and put inside a vented flask. Water is added to the flask until it just covers the sample, and the volume of sample and water is recorded. Then the sample is removed and the volume of water alone recorded. From the mass of the sample and the difference in volumes, the value of R may be calculated.
Property V: Sample of X is melted and put into a reservoir from which a very thin tube leads down. The rate at which X flows out of the tube is measured, and from this rate the value of V may be calculated.
Answers
Answer:
The property P measured, was a chemical property of X; its acidity
The property D measured, was a physical property of X; its density
The property V measured, was a physical property of X; it's viscosity probably.
Explanation:
1. The property V that was measured is the acidity of X. The acidity of X is a measure of the concentration of hydrogen ions present in it. When drops of another solution, containing dissolved sodium hydroxide, are slowly added, and a pH indicator is used to determine when the sodium hydroxide has completely reacted with X, the procedure determines the concentration of X that would neutralize the base, sodium hydroxide.
2. The property of X determined in the step is the density of X, a physical property. First the volume of X was determined by the displacement method. Then the density of X is calculated from its mass and volume.
3. Since during the determination of the property V, there was no alteration done to the chemical constitution of X, the property thus determined is a physical property. Melting of the sample is a physical change and likewise its flow rate through a very thin tube.
11.0g of sulfur hexafluoride gas to liters of sulfur hexafluoride.
hurry pls
Answers
Divide the weight with 1,000 times the component or material's density to the conversion from grammes to litres. Sulfur hexafluoride in litres is produced from 11.0g of sulphur hexafluoride gas. 0.0753138778434317.
Does 1 kilogramme equal 1 litre?What volume of water is equivalent to one kilogramme. When pure water attains a maximum density of one kg/l at a temperature around 39.2 °F or 4 °C, it has a volume of 1 litre. 1 kilogramme of water is somewhat more than 1 litre at higher temperatures.
How much mol of S are there in 4.0 g of SF6?As there is one mole of SF in every six moles, there are now 0274 moles in total. After solving, we get the result of.164 mole of fluorine. Adding 1 mole will also have 6 moles od fluorine, giving us 0.0274 moles in s f, 6, corresponding to 6 times in 0.0274 mole of chlorine. Hence, in 4 grammes.
To know more about hexafluoride visit:
https://brainly.com/question/24186669
#SPJ1
How many grams of benzene are present if it took 13500 joules for benzene to boil?
Answers
The question is confusing, but if you are saying that the heat of vaporization is 393 joules per gram, and 30 grams condense, then the answer is simply 30 * 393 = 11790 joules.
In a chemical reaction, if one knows the mass of A, one could determine:
1) the mass of any other reactant and product
2) only the mass of B
3) only the mass of C
4) only the mass of A
Answers
If one knows the mass of A, they may calculate the mass of any other reactant and product in a chemical reaction.
How can the mass relationship in a chemical reaction be determined?Find out how many moles each reactant has. To identify which reactant is limiting, compare the mole ratios of the reactants with the ratio in the balanced chemical equation. Determine how many moles of the limiting reactant can be converted into the product.
When the mass of the limiting reactant is known, how does one calculate the mass of the product?Calculate the maximum number of moles of product that can be produced from the limiting reactant using mole ratios. Add the product's molar mass to the number of moles.
To know more about chemical reaction visit:-
brainly.com/question/29762834
#SPJ1
a fisherman in a boat is drinking a of hot coffee. the large lake below his boat is full of cold water. which statement is an accurate comparison of the lake water and the coffee?
Answers
Answer:
see below
Explanation:
The lake will have more total thermal energy but the particles in the coffee will be moving faster.
A comparison of hot coffee and cold lake is, the heat from the coffee will be absorbed by the cold lake through convection method of heat transfer.
Conservation of energy
The principle of conservation of energy states that the total energy of an isolated system is always conserved.
Heat lost by the hot coffee = Heat absorbed by the cold lake
Heat transfer processHeat from the coffee will be absorbed by the cold lake through convection method of heat transfer.
Thus, a comparison of hot coffee and cold lake is, the heat from the coffee will be absorbed by the cold lake through convection method of heat transfer.
Learn more about heat transfer here: https://brainly.com/question/12072129