Which of the following elements is the most reactive metal?
a. Aluminum
b. Zinc
c. Carbon
d. Lithium
Answers
Answer:
D
Explanation:
lithium is an alkali metal. alkali metals are very very reactive
Related Questions
Cyclohexene reacts with bromine to yield 1.2-dibromocyclohexane. Molecules of the product would: A) be a racemic form and, in their most stable conformation, they would have both bromine atoms equatorial. B) be a racemic form and, in their most stable conformation, they would have one bromine atom equatorial and one axial C) be a meso compound and, in its most stable conformation, it would have both bromine atoms equatorial D) be a meso compound and, in its most stable conformation, it would have one bromine atom equatorial and one axial E) be a pair of diastereomers and, in their most stable conformation, one would have the bromines equatorial and axial, and thether would have the bromines equatorial and equatorial
Answers
Be a racemic form and, in their most stable conformation, they would have one bromine atom equatorial and one axial.
This is because the addition of bromine to cyclohexene forms a mixture of both cis and trans isomers of 1.2-dibromocyclohexane. In the most stable conformation, one bromine atom will be equatorial and the other will be axial, as this minimizes the steric hindrance caused by the bulky bromine atoms.
The product will be a racemic mixture because both enantiomers will be formed in equal amounts. Option A is incorrect because both bromine atoms cannot be equatorial in the most stable conformation. Option C is incorrect because a meso compound must have a plane of symmetry, which is not present in 1.2-dibromocyclohexane. Option D is incorrect for the same reason.
To know more about racemic visit :-
https://brainly.com/question/31665891
#SPJ11
How long would it take to deposit 15.0 g copper metal at the cathode of an electrolysis cell running with a current of 150 mA
Answers
It would take 10,292 seconds to deposit 15.0 g copper metal at the cathode of an electrolysis cell running with a current of 150 mA.
The amount of copper deposited at the cathode of an electrolysis cell is directly proportional to the electric charge passing through the cell. The charge Q (in coulombs) can be calculated by multiplying the current I (in amperes) by the time t (in seconds), and the amount of copper deposited can be calculated using the formula:
m = Q * (M / (n * F))
where m is the mass of copper deposited (in grams), M is the molar mass of copper (63.55 g/mol), n is the number of electrons involved in the reduction of copper ions (2 in this case), and F is the Faraday constant (96,485 C/mol).
So, we can rearrange this formula to solve for the time t:
t = m * n * F / (I * M)
Plugging in the given values, we get:
t = 15.0 g * 2 * 96,485 C/mol / (0.150 A * 63.55 g/mol)
t = 10,292 seconds
Therefore, it would take 10,292 seconds or approximately 2.86 hours to deposit 15.0 g of copper metal at the cathode of an electrolysis cell running with a current of 150 mA.
To know more about the electrolysis cell refer here :
https://brainly.com/question/31473489#
#SPJ11
copper metal is placed in a solution of silver nitrate
Answers
The balanced chemical equation for the reaction between copper and silver nitrate is
Cu(s) + 2AgNO₃(aq) → 2Ag (s) + Cu(NO₃)₂ (aq)
In the above reaction, Cu is a reactant, AgNO₃ is another reactant
When both are mixed they make a reaction where there are formed Ag and Cu(NO₃)₂.
This is a redox reaction where:
Cu is reduced ⇒ Cu → Cu²⁺ + 2e⁻
Ag⁺ is oxidized ⇒ Ag⁺ + 1e⁻ → Ag
To balance the reaction, we multiply the second half reaction by 2
Cu → Cu²⁺ + 2e⁻
(Ag⁺ + 1e⁻ → Ag) .2 ⇒ 2Ag⁺ + 2e⁻ → 2Ag
When we sum both, we cancel the electrons:
Cu + 2Ag⁺ + 2e⁻ → 2Ag + Cu²⁺ + 2e⁻
Balanced reaction is:
Cu(s) + 2AgNO₃(aq) → 2Ag (s) + Cu(NO₃)₂ (aq)
To know more about copper here
https://brainly.com/question/32039651
#SPJ4
The complete question should be
Write the chemical equation for this process: ""Copper metal is placed in a solution of silver nitrate. This produces silver metal and copper nitrate solution.""
a light bulb consumes energy at a rate of 80.0 joules per second. how long in seconds will it take for the light bulb to consume 2.30x10
Answers
The light bulb will take approximately 2.9 x 10² seconds to consume 2.30 x 10² joules of energy.
To calculate the time it takes for the light bulb to consume a given amount of energy, we can use the formula:
Time (in seconds) = Energy Consumed / Energy Consumption Rate
Given that the energy consumption rate of the light bulb is 80.0 joules per second, and we want to find the time it takes for the light bulb to consume 2.30 x 10² joules of energy, we can substitute these values into the formula:
Time = (2.30 x 10² joules) / (80.0 joules per second)
Time = 2.875 x 10² seconds
Time ≈ 2.9 x 10² seconds
learn more about Energy here:
https://brainly.com/question/31434118
#SPJ11
Which example involves the transformation of chemical enegy directly to light energy?
1.A glow stick
2.Photosynthesis
3.Windmill
4.A hydroelectric dam
Answers
A glow stick is an example of a device that directly converts chemical energy into light energy.
A) A glow stick generates luminance, or light energy, using chemical energy from the compounds contained within it. When the stick is broken or twisted, the chemicals inside it are released and, through a chemical reaction, chemical energy is transformed into light energy.
B) plants employ light energy during photosynthesis to create oxygen and energy in the form of sugar (glucose).
C) The wind serves as the energy source for a windmill, and when the rotor blades revolve, the wind energy is transformed into mechanical energy. Then, electrical energy is created from that mechanical energy. The light energy produced by these electrical currents is subsequently employed in lamps. As a result, the chemical energy is not changed directly.
D) Water provides mechanical energy to a hydroelectric dam. When the water reaches the turbine, the gravitational potential energy that it had because of its height is transformed into kinetic energy. Then electrical energy is created from this kinetic energy.
Therefore, Glow Stick is the sole example where chemical energy is transformed into light energy in this manner.
Learn more about energy conversion here:
brainly.com/question/11234965?referrer=searchResults
#SPJ1
different liquids have widely differing vapor pressures at the same temperature. discuss this range of vapor pressures in terms of entropy and enthalpy with regard to the attractive intermolecular (im) forces between molecules and the degree of dispersal between the liquid and gaseous states.
Answers
The range of vapor pressures in different liquids at the same temperature can be explained in terms of entropy and enthalpy. Liquids with weaker intermolecular forces tend to have higher vapor pressures due to higher entropy, while liquids with stronger intermolecular forces tend to have lower vapor pressures due to lower enthalpy.
Different liquids have widely differing vapor pressures at the same temperature due to variations in their intermolecular forces and the degree of dispersal between the liquid and gaseous states.
The vapor pressure of a liquid is the pressure exerted by its vapor when it is in equilibrium with its liquid phase at a given temperature. It depends on the attractive intermolecular forces between the molecules and the extent to which the liquid molecules can escape into the gas phase.
1. Entropy: Entropy is a measure of the disorder or randomness in a system. Liquids with weaker intermolecular forces have higher entropy because the molecules are more free to move and disperse. These liquids tend to have higher vapor pressures at a given temperature because the molecules can more easily overcome the attractive forces and enter the gas phase.
2. Enthalpy: Enthalpy is a measure of the total energy of a system. Liquids with stronger intermolecular forces have lower enthalpy because more energy is required to break the attractive forces and convert the liquid into a gas. These liquids tend to have lower vapor pressures at a given temperature because fewer molecules can overcome the attractive forces and enter the gas phase.
For example, consider two liquids: water and ethanol. Water has strong hydrogen bonding between its molecules, resulting in lower entropy and higher enthalpy. As a result, water has a lower vapor pressure at a given temperature compared to ethanol, which has weaker intermolecular forces. Ethanol has higher entropy and lower enthalpy, allowing more molecules to escape into the gas phase and resulting in a higher vapor pressure.
Learn more about vapor pressures here:-
https://brainly.com/question/34135527
#SPJ11
experiment 3: which unknown solution contained more copper(ii) sulfate? this cannot be determined from the experiments performed here. copper solution
Answers
Based on the given information, it cannot be determined which unknown solution contained more copper(ii) sulfate. The experiments performed only involved a copper solution and did not provide any comparative measurements or quantities of copper(ii) sulfate in the unknown solutions. Therefore, further testing or analysis would be necessary to determine which unknown solution contained more copper(ii) sulfate.
To determine the amount of copper(II) sulfate in a solution, one could perform a quantitative analysis such as titration or gravimetric analysis. Without such experiments, it is impossible to determine which solution contained more copper(II) sulfate.The given information only suggests that there were two unknown solutions, and one of them contained copper(II) sulfate. Therefore, the only conclusion that can be made is that the copper solution contained copper(II) sulfate.
Learn more about copper(II) sulfate here, https://brainly.com/question/15336195
#SPJ11
Seleccione una actividad humana y el impacto generado debe realizar un resumen sobre la actividad seleccionada y dos medidas de prevención para evitar la alteración de los ciclos.
Answers
Answer:
Industrial activity increases the emission of greenhouse gases in the atmosphere, especially carbon dioxide (CO2), and it negatively affects climate by increasing global warming.
Two actions to face the global warming:
1- To substitute the use of fossil fuels by alternative clean energy sources such as, for example, hydroelectric, geothermal, solar and wind energy resources.
2- The government's policies decided to develop electric cars and to stimulate healthy habits such as walking instead of the use of conventional fossil-fueled transport modes.
The half-life of hafnium-156 is 0.025 s. How long will it take a 560 g sample to decay to 35 g?
Answers
Answer:
0.1 seconds
Explanation:
s=seconds
Decay by a half-life is exponential since you're taking away half of the previous life every time. The reduction in hafnium is not constant. You don't take away the same amount of hafnium from the existing hafnium every 0.025 s since the reduction in hafnium decreases by half. Every 0.025 s, you take away half of what you've taken in the previous 0.025 s.
560/35 = 16
Half-life means 1/2 of the previous life of hafnium when time forwards 0.025 s. However, if you go back 0.025 s in time, you double the life by 2.
2^x = 16 ==> solve for x
x = 4 ==> 2^4 = 16
Now multiply 4 and 0.025 to get the total time:
4 * 0.025 = 0.100 ==> 0.1 seconds
To learn more about decay, click here:
https://brainly.com/question/27144626
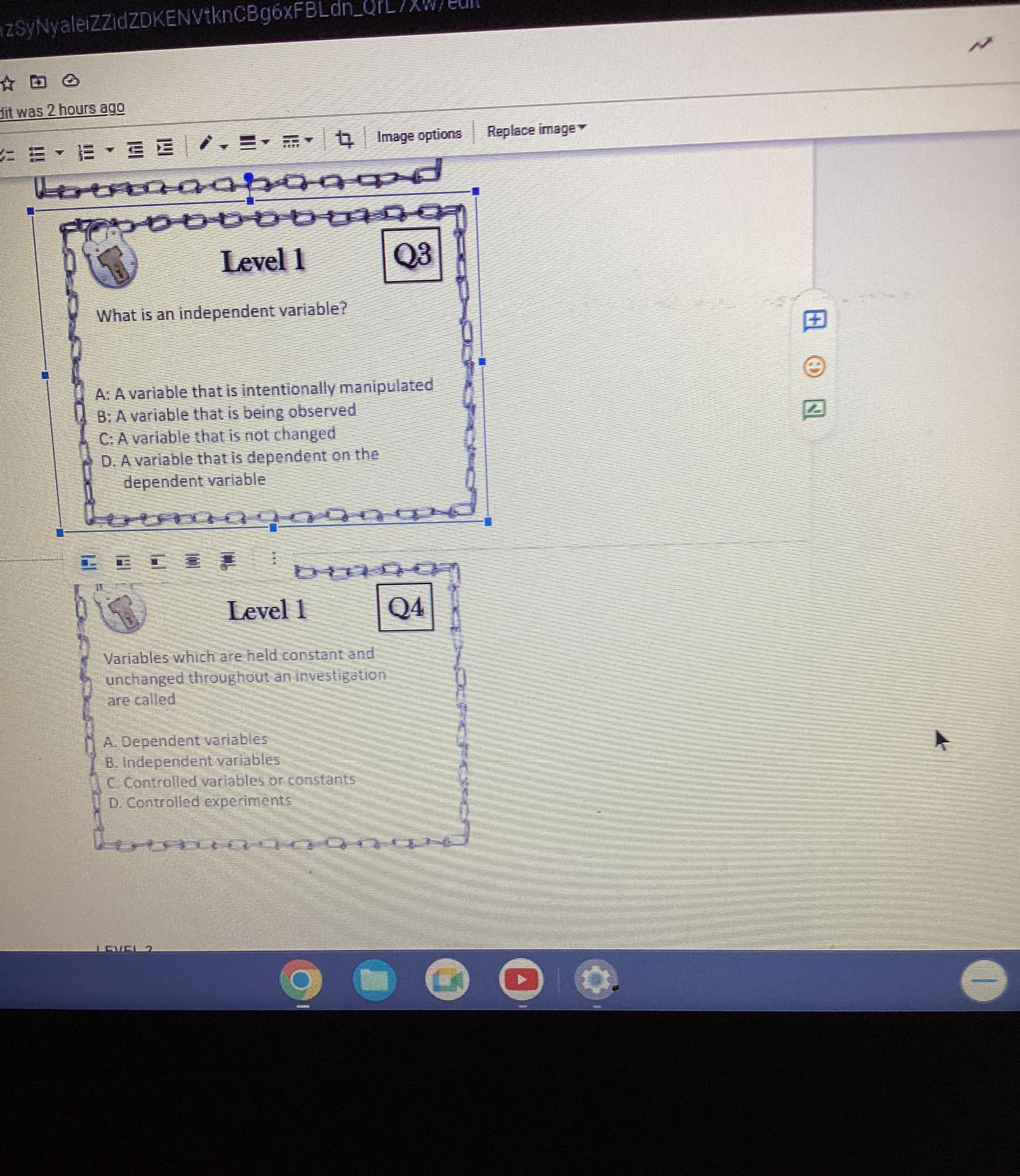
Level 1
What is the first step in completing the
scientific method?
A: Experimentation
B: Forming a hypothesis
C: Analyzing data
D: Making observations
Q1
Level 1
Q2
Which of the following is not true about
a hypothesis?
A: It is an explanation for an observation
B: It must restate the question
1. C. It is testable
D. It can be written as an if/then statement,
Answers
Answer: Level 1's answer is D/ Making observations. The second one is it must restate the question/ B. No. 3:variable (often denoted by x ) whose variation does not depend on that of another. No.4 IS Controlled Vari
Explanation:
What’s science in science
Answers
Answer:
Science is the pursuit and application of knowledge and understanding of the natural and social world following a systematic methodology based on evidence. Scientific methodology includes the following: Objective observation: Measurement and data (possibly although not necessarily using mathematics as a tool)
Explanation:
Answer:
Modern science is typically divided into three major branches that consist of the natural sciences (e.g., biology, chemistry, and physics), which study nature in the broadest sense; the social sciences (e.g., economics, psychology, and sociology), which study individuals and societies; and the formal sciences
plllllz its 100 and i give brain list

Answers

Answer:
Your answer is the on with the clownfish and the anemone
Explanation:
Hope this helps!
Smoking cigarettes is a lifestyle choice that may lead to cardiovascular disease.
Some people who have never smoked cigarettes still have cardiovascular disease.
Give two other factors that may contribute to the occurrence of cardiovascular
disease.
Answers
Answer:
High blood pressure. High blood pressure (hypertension) is one of the most important risk factors for CVD.
High cholesterol - High-fat diet.
3. How many moles are present in 100 g of Ca(NO3)2?
PLEASEEE HELP ASAPP
Answers
0Answer: 0.6094
Explanation:
no of moles = mass / molar mass = 100/164.088= 0.6094 mole
Answer:
0.609 moles
Explanation:
mass in g ÷ atomic mass = moles
Ca(NO₃)₂ = 1 Ca 40.078 amu
2 N 28.0134 amu
+ 6 O 95.994 amu
____________________
164.0854 amu
100 g ÷ 164.0854 amu = 0.609 moles
three significant digits
1. Mountains are most likely formed
a. when glaciers melt.
b. from earthquakes.
C. when land sinks.
d. where tectonic plates collide.
Answers
select the statements that correctly describe the half-life of radioisotopes. select all that apply. multiple select question. a. radioisotopes used in medicine have relatively short half-lives.
b. if the half-life and amount of sample are known, the pressure and temperature are still needed to calculate the decay of the sample.
c. increasing the temperature of a radioisotope increases the rate of nuclear decay.
d. many naturally occurring radioisotopes have long half-lives.
Answers
The correct statements about the half-life of radioisotopes are:
a. Radioisotopes used in medicine have relatively short half-lives.
d. Many naturally occurring radioisotopes have long half-lives.
a. Radioisotopes used in medicine often have short half-lives because they need to decay and become less radioactive within a reasonable time frame. This allows for safer handling and reduces the duration of radiation exposure to patients.
b. The half-life of a radioisotope is a characteristic property and does not depend on pressure or temperature. The decay rate is determined solely by the nature of the radioisotope itself, so the pressure and temperature are not required to calculate the decay of the sample.
c. Increasing the temperature of a radioisotope does not affect the rate of nuclear decay. The decay process is governed by nuclear interactions and is not influenced by temperature changes.
d. Many naturally occurring radioisotopes have long half-lives, which means they decay at a slower rate. This is why they can be found in significant quantities in natural sources such as rocks, minerals, and the environment.
To learn more about radioisotopes click here:
brainly.com/question/28142049
#SPJ11
What mass of methanol (CH3OH) is produced when 140.1 g of carbon
monoxide reacts with 12.12 g of hydrogen?
CO(g) + 2H2(g) → CH2OH()
A. 160.2g
B. 96.12 g
C. 6.06 g
D. 192.2g
Answers
Answer:
B. 96.12 g
Explanation:
To solve this question we must convert each reactant to moles. Using the balanced equation we can find limiting reactant. With moles of limiting reactant we can find the moles of methanol and its mass:
Moles CO -Molar mass: 28.01g/mol-
140.1g CO * (1mol / 28.01g) = 5.0 moles CO
Moles H2 -Molar mass: 2.01g/mol-
12.12g * (1mol / 2.01g) = 6.03 moles H2
For a complete reaction of 5.0 moles of CO are required:
5.0moles CO * (2mol H2 / 1mol CO) = 10.0 moles of H2 are required
As there are just 6.03 moles, H2 is limiting reactant.
The moles of methanol produced are:
6.03 moles H2 * (1mol CH3OH / 2mol H2) = 3.015 moles CH2OH
Mass CH3OH -Molar mass32.04g/mol-:
3.015 moles CH2OH * (32.04g/mol) =
96.6g CH3OH ≈ B. 96.12 g
classify nacl as a neutral, acidic, or basic salt.
Answers
NaCl is an example of a neutral salt.
Why is NaCl a neutral salt?
Sodium chloride (NaCl) is a neutral salt because it is composed of both a cation (Na+) and an anion (Cl-) that have equal and opposite charges. The positive charge of the sodium ion (Na+) is exactly balanced by the negative charge of the chloride ion (Cl-), resulting in a neutral compound.
When NaCl dissolves in water, the water molecules surround the ions and separate them from each other. The positively charged sodium ions (Na+) are attracted to the negatively charged oxygen atoms in water molecules, and the negatively charged chloride ions (Cl-) are attracted to the positively charged hydrogen atoms in water molecules. This process is called hydration or solvation.
Learn more about neutral salt:https://brainly.com/question/3103303
#SPJ1
I need to someone to explain the whole being of Chemistry. I do not understand a single thing, nothing makes sense to me. Literally, I'm going to fail this class because I do NOT understand a single thing taught to me. Maybe it's because I was mistakenly put into Chemistry Pre AP instead of on level but it's so hard and- I just do not get it.
Answers
Answer:
Explanation:
In Pre-AP Chemistry, the development of models to explain their macroscopic observations is a primary means through which students develop an understanding of the molecular world.
You will be forced to think and apply concepts to new situations, and even derive your own theories from application. This is excellent preparation for the higher levels of thinking required in college.
Chemistry, the science that deals with the properties, composition, and structure of substances (defined as elements and compounds), the transformations they undergo, and the energy that is released or absorbed during these processes.
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
2. If you put 156. 32g barium hydroxide into this reaction, how much aluminium hydroxide can be
produced?
Answers
When 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced, based on the balanced chemical equation and stoichiometry.
To determine the amount of aluminum hydroxide that can be produced when 156.32 g of barium hydroxide is reacted, we need to consider the balanced chemical equation for the reaction and use stoichiometry.
The balanced chemical equation for the reaction is:
Ba(OH)2 + 2AlCl3 → 2Al(OH)3 + 3BaCl2
From the balanced equation, we can see that for every 1 mole of Ba(OH)2, 2 moles of Al(OH)3 are produced.
First, we need to calculate the number of moles of barium hydroxide (Ba(OH)2) in 156.32 g:
Molar mass of Ba(OH)2 = (137.33 g/mol + 2(16.00 g/mol + 1.01 g/mol)) = 171.34 g/mol
Moles of Ba(OH)2 = mass / molar mass = 156.32 g / 171.34 g/mol = 0.911 mol
Now, using the stoichiometry of the balanced equation, we can determine the moles of aluminum hydroxide (Al(OH)3) produced:
Moles of Al(OH)3 = 2 × Moles of Ba(OH)2 = 2 × 0.911 mol = 1.822 mol
Finally, to convert the moles of aluminum hydroxide to grams, we need to multiply by the molar mass of Al(OH)3:
Molar mass of Al(OH)3 = (26.98 g/mol + 3(16.00 g/mol + 1.01 g/mol)) = 78.00 g/mol
Mass of Al(OH)3 = Moles of Al(OH)3 × molar mass = 1.822 mol × 78.00 g/mol = 142.34 g
Therefore, when 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced.
For more such questions on barium hydroxide visit;
https://brainly.com/question/29344018
#SPJ8
What kinds of intermolecular forces are present in a mixture of potassium chloride and water?
Answers
The kinds of intermolecular forces which are present in a mixture of potassium chloride and water are ion-dipole forces and hydrogen bonds.
What is Hydrogen bond?This is referred to as a special type of dipole-dipole attraction between molecules. It is also known as a weak bind and exists between a proton in one molecule and an electronegative atom in the other.
Ion-dipole forces on the other hand is referred to as the type of force which commonly present in compounds such as the solutions of ionic compounds in polar liquids which depicts the mixture of potassium chloride and water.
Read more about Hydrogen bond here https://brainly.com/question/1426421
#SPJ1
Is the following equation balanced or unbalanced? 4Na + O2 ➡️ Na2O
Answers
The given chemical equation is unbalanced.
What is an unbalanced equation ?An unbalanced equation is a chemical equation that does not have the same number of atoms of each element on both sides of the equation. In other words, the number of atoms of each element present in the reactants (the substances being reacted) is not the same as the number of atoms of each element in the products (the substances produced by the reaction).
As one can see from the equation, there are more Sodium ( Na ) atoms on the left side of the equation than on the right side. The equation is therefore unbalanced.
Find out more on unbalanced equations at https://brainly.com/question/27692530
#SPJ1
A football player runs 80 yards for a touchdown! It takes him 20 seconds. What is his speed?

Answers
Answer:
4 yards/second is the answer
Answer:
the answer is 4 yards per second
Explanation:
80 yards in 20 seconds
20+20+20+20=80
there are 4, 20s to make 80
in other words...
80÷4=20
Describe how glacier mice are complete ecosystems, even though they are tiny.
GIVING BRAINIEST
Answers
Answer: The clumps, known as glacier mice, have been found to contain miniature ecosystems
Explanation: Because glaciers are in constant, if slow, motion and are frequently blasted by strong winds.
The clumps, known as glacier mice, have been found to contain miniature ecosystems. And even in freezing temperatures, scientists found, the inhabitants manage to thrive. In high winds glacier mice, which form when clumps of dust and organic debris develop a layer of moss over time, hop across vast sheets of ice.
What organisms live in glacier mice?These included springtails (small insect-like animals), water bears (also known as tardigrades, the only animals known to have survived in space), and roundworms. Globally, glacier mice are rare.
Learn more about glacier mice here: brainly.com/question/22404059
#SPJ2
Sodium chloride is NaCl, and zinc chloride ZnCl₂. Why are there more chloride ions in the zinc compound?
Answers
Answer:
Zinc chloride (ZnCl2) has more chloride ions compared to sodium chloride (NaCl) because the zinc ion has a +2 charge, while the sodium ion has a +1 charge.
Chlorine (Cl) is a halogen element with a -1 charge when it gains an electron to form an ion. To form a neutral compound, the number of positive and negative charges must be balanced.
In NaCl, the sodium ion has a +1 charge and can only bond with one negatively charged chloride ion, which has a -1 charge. Therefore, the formula for sodium chloride is NaCl, with one sodium ion and one chloride ion.
In ZnCl2, the zinc ion has a +2 charge, and it requires two negatively charged chloride ions, each with a -1 charge, to form a neutral compound. Therefore, the formula for zinc chloride is ZnCl2, with one zinc ion and two chloride ions.
Thus, the reason why there are more chloride ions in the zinc compound is because the zinc ion has a higher charge compared to the sodium ion, and it requires two chloride ions to balance its charge, whereas sodium ion requires only one chloride ion.
why is it important that the level of the sea water does not go above the level of the filter paper
Answers
Answer:
The water (the substance) that passes through the filter paper is called the filtrate. If your mixture is a solution, such as salty water, then filtering will not separate the salt from the water. Instead, by heating the soluton the solvent (water) evaporates leaving the solid (salt) behind.
Explanation:
What volume of oxygen (02) gas is equal to 49.5 g 02 gas at STP?
Answers
Answer:
The volume of two moles of oxygen at STP is
Assuming that the gas is at standard temperature and pressure (STP), one mole of any gas occupies 22.4 L . This means the number of moles of O2 is 222.4=0.089 mol .
Explanation:
At the beginning of the day, it was 4°. Throughout the day, the temperature decreased 13°. What was the temperature (in degrees) at the end of the day?
Answers
Answer: -9°
Explanation:
4 - 13 = - 9°
HELP ASPAP!!!!!!!!PLEASE PLEASE
Answers
Answer:
what do you need help with?
Explanation: