Answers
Explanation:
i think the answer is....b
The diagram that represents single replacement reaction is:
b.
Single replacement reaction:This reaction is also known as displacement reaction. It is a reaction in which one element is substituted for another element in a compound. When a replacement reaction occurs, a new aqueous compound and a different pure element will be generated as products. For example:
\(2HCl(aq)+Zn(s)--->ZnCl_2(aq)+H_2(g)\)
Thus, option b is correct.
Find more information about displacement reaction here:
brainly.com/question/20690229
Related Questions
please answer this 1. rana wants to buy shirts for summer.should she buy cotton shirts or shirts made of synthetic material ? advise rana, give ur reason...
Answers
Answer:
Cotton Shirts
Explanation:
I would think that the cotton shirts would be more appropriate form of of clothing for summer.
Cotton has tiny pores that enable the sweat to evaporate off your skin, and hence keep you cooler. At the same time it absorbs this sweat, allowing easy evaporation. Synthetic fibers hold heat in, and do not allow sweat to evaporate.
It would be that Rana should buy cottons shirts for summer.
what is the percent composition of hydrogen in beryllium hydride(BeH2) if 69.6g of beryllium (Be) react with 15.6 g of hydrogen to produce 85.2 g of BeH2
Answers
The percent composition of hydrogen is 18.3%.
What is percent composition?The term percent composition refers to the percentage of a particular component in a compound. It is contained as the ratio of the mass of that component to the total mass multiplied by 100.
From the law of conservation of mass, total mass of beryllium hydride(BeH2) = 85.2 g
Mass of hydrogen = 15.6 g
Percent composition of hydrogen = 15.6 g/ 85.2 g × 100/1 = 18.3%
Learn more about percent composition: https://brainly.com/question/12247957
Gold has a specific heat of 0.129 J/g °C. How many joules of heat energy are
required to raise the temperature of 23 grams of gold from 22 °C to 98
°C?
Look at picture
ASAP please will mark as brainlist don’t got much time

Answers
Answer:
210.657J
Explanation:
joules of heat energy needed= 23×0.129×(93-22)
=210.657J
what is the formula for caculating time
Answers
Answer: Time = Distance ÷ Speed
Identify the type of energy this object possesses. A girl roller-skating Kinetic energy Potential energy
Answers
A girl roller-skating has kinetic energy.
Kinetic energy is the energy of motion. It is the energy possessed by an object due to its movement. In this case, the girl roller-skating has kinetic energy because she is moving.
Potential energy is the energy an object possesses due to its position or configuration. It is the energy an object has stored within it, ready to be released. An object at rest has potential energy because it has the potential to be set in motion and does work.
So, in this case, the girl roller-skating has kinetic energy because she is moving, and not potential energy because she is not at rest.
To learn more about kinetic energy, check out https://brainly.com/question/24933254
sodium and oxygen react to produce sodium oxide how many moles of oxygen are needed to produce 11.15 g of sodium oxide
Answers
Answer:
0.0899 moles of oxygen (O2).
Explanation:
What is given?
Mass of sodium oxide (Na2O) = 11.15 g.
Molar mass of Na2O = 62 g/mol.
Step-by-step solution:
First, let's state the balanced chemical equation:
\(4Na+O_2\rightarrow2Na_2O.\)Let's calculate the moles of Na2O that are in 11.15 g of Na2O, using its molar mass:
\(11.15\text{ g Na}_2O\cdot\frac{1\text{ mol Na}_2O}{62\text{ g Na}_2O}=0.1798\text{ moles Na}_2O.\)Now that we have the moles of Na2O, let's do the stoichiometry: you can see in the chemical equation that 1 mol of oxygen (O2) reacted produces 2 moles of Na2O, so by doing a rule of three based on this data, the calculation will look like this:
\(0.1798\text{ moles Na}_2O\cdot\frac{1\text{ mol O}_2}{2\text{ moles Na}_2O}=0.0899\text{ moles O}_2.\)The answer is that we need 0.0899 moles of oxygen (O2) to produce 11.15 g of sodium oxide.
how is thermal energy apart of weather changing
Answers
Answer:
Heat, in the form of thermal energy, naturally moves from warmer substances to colder ones. When the ocean is warmer than the atmosphere, it transfers heat — through conduction and radiation — to make the air warmer. ... This redistributes thermal energy and causes changes in the weather.
What is the pneumonic of the metric line
Answers
Answer:
Explanation:
Mnemonic Device: Kings Hate Dragons Because Dragons Can't Make Money. Explanation: to remember the Metric prefixes, or the decimal ladder, and base unit (Kilo, Hecto, Deca, Base, Deci, Centi, Milli, Micro).
16. What would happen to the air in an empty container if you crumpled the container into a smaller shape? A. The gas changes shape to fit the container, but not its volume. B. The gas changes shape and volume to fit the container. C. The gas does not change shape or volume to fit the container. D. Atoms in water have stronger bonds than atoms in hydrogen peroxide.
Answers
Answer:
B. The gas changes shape and volume to fit the container.
Explanation:
Fluids are substances which can flow and which do not have a definite shape but take up the shape of their container. Gases are fluids and so do not have a fixed shape. Gases also do not have fixed volumes but are compressible since the distances between molecules are large due to the negligible force of attraction between the molecules.
Therefore, if a container filled with air is crumpled, the molecules of the gas will take up the new shape of the container. Also, since air is compressible, its volume will change to the new volume of the container.
What is the percent yield of lithium hydroxide from a reaction of 7.40 g of lithium with 10.2 g of water? The actual yield was measured to be 12.1 g.
2Li(s) + 2H2O(l) = 2LiOH(aq) + H2(g)
Select one:
a. 72.5%
b. 53.1%
c. 89.3%
d. 47.4%
Answers
Answer: d. 47.4%
What is the percent yield of lithium hydroxide from a reaction of 7.40 g of lithium with 10.2 g of water? The actual yield was measured to be 12.1 g.
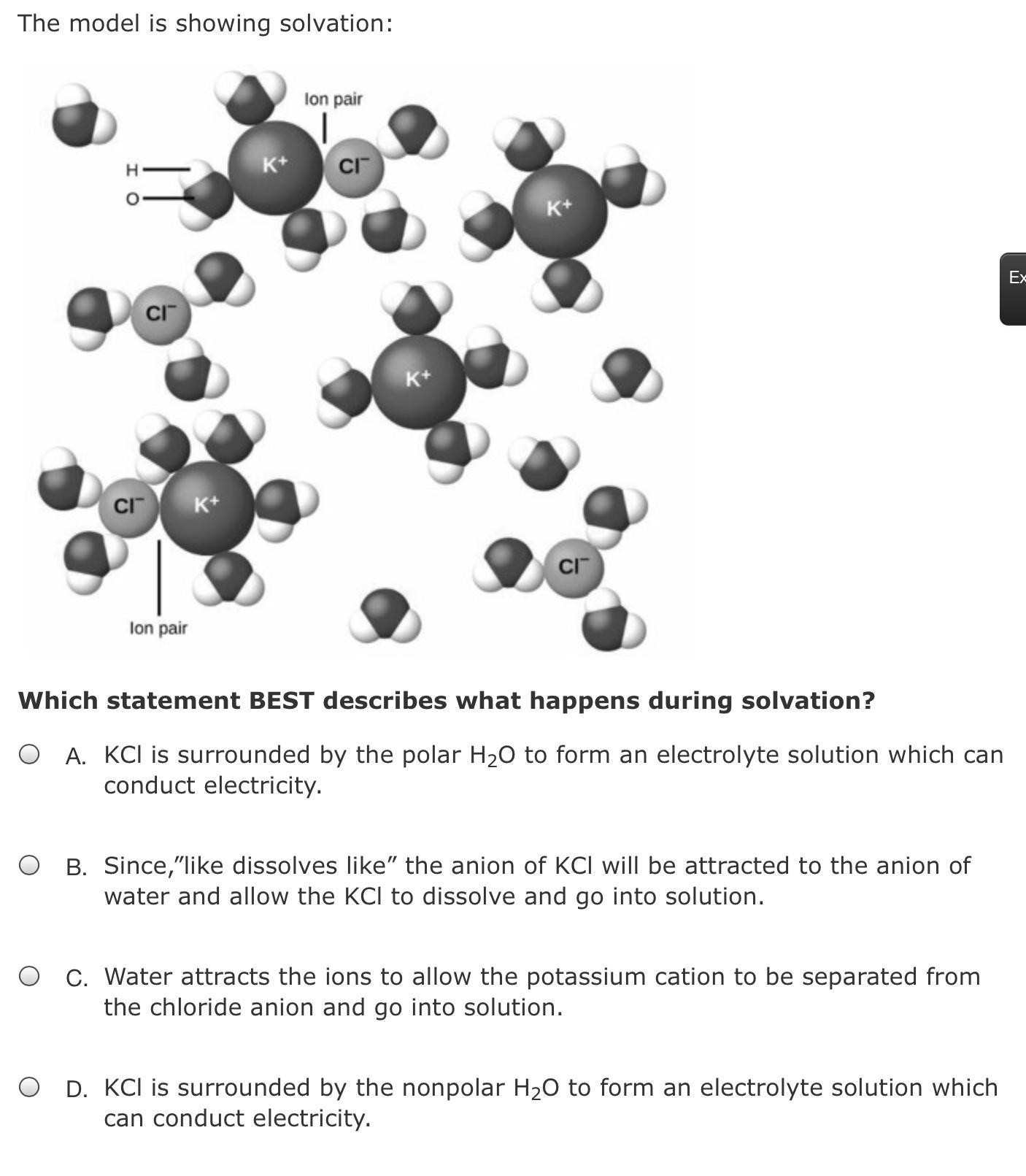
Which statement BEST describes what happens during solvation?
Answers
Answer:
A
Explanation:
The statement best describes solvation " KCl is surrounded by the polar water molecule to form an electrolyte solution which can conduct electricity."
What is solvation?The act of attracting and associating particles of a solvent mostly with molecules as well as ions of a solute would be known as solvation.
What is electrolyte?A material that contains ions and has been electrically conducting due to the mobility of the ions but still doesn't conduct electrons was called an electrolyte.
Whenever the solvent would be in the water, the process is referred to as hydration. The process of solvation shows the combination of solvent and solute molecules. A solute molecule is usually surrounded with solvent molecules that are organized in a special manner during solvation.
Therefore, the correct answer will be an option (A)
To know more about solvation and electrolyte
https://brainly.com/question/14356923
#SPJ3
80 calories must be lost to make water change into ice at 0° C. True False
Answers
How many moles are there in 500cm3 of a 0.5mol/dm³ solution of sulfuric acid?
Answers
Answer:
0.25moles
Explanation:
There are 1000\(cm^{3}\) for 1\(dm^{3}\)
Therefore in 1000\(cm^{3}\) of 0.5 mol/\(dm^{3}\) solution has = 0.5 moles
Therefore 500\(cm^{3}\) contains = 0.5/1000 x 500 = 0.25moles
The number of moles of sulfuric acid in 0.5 mol/dm³ solution is equal to 0.25 mol.
What is the molarity?The concentration of the solution can be determined as the number of moles of a solute in per unit volume of a solution is known as molarity or molar concentration.
The Molarity of the solution is calculated in the following formula:
Molarity (M) = Moles (n)/Volume of the Solution (in L)
Now if we have to find the number of moles of solute in the solution, the formula becomes:
Number of moles of solute (n) = Molarity (M) × Volume of the Solution
Given, the concentration of the sulfuric acid solution = 0.5 mol/dm³
The volume of the solution, V = 500 cm³
As we know, 1 dm³ = 10³ cm³, the volume of solution = 0.5 dm³
The number of moles of the sulfuric acid = M × V
= 0.5 × 0.5 = 0.25 mol
Therefore, 0.25 moles of sulfuric acid in 500cm3 of a 0.5mol/dm³ solution of sulfuric acid.
Learn more about molarity, here:
https://brainly.com/question/8732513
#SPJ2
A student was measuring the time it took for an experiment to take place. They found the time to be 40 seconds. However, they had started timing a few seconds after the experiment had started, and the actual time was 43 seconds. What is the percent error?
Answers
Answer:
9.3%
Explanation:
Percent error = (actual value - experimental value / actual value) × 100
In this question, the actual value is 43seconds while the experimental value is 40 seconds
Hence,
%error = (43 - 40 / 43) × 100
%error = (4/43) × 100
%error = 0.093023 × 100
%error = 9.3%
How many molecules are in 7.32 moles of sulfur dioxide?
Answers
Answer:
4.41 × 10²⁴ molecules SO₂
General Formulas and Concepts:
Atomic Structure
CompoundsMolesStoichiometry
Using Dimensional AnalysisExplanation:
Step 1: Define
[Given] 7.32 moles SO₂
[Solve] molecules SO₂
Step 2: Identify Conversions
Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.
Step 3: Convert
[DA] Set up: \(\displaystyle 7.32 \ moles \ SO_2(\frac{6.022 \cdot 10^{23} \ molecules \ SO_2}{1 \ mol \ SO_2})\)[DA] Multiply [Cancel out units]: \(\displaystyle 4.4081 \cdot 10^{24} \ molecules \ SO_2\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
4.4081 × 10²⁴ molecules SO₂ ≈ 4.41 × 10²⁴ molecules SO₂
Answer:
4.41 × 10²⁴ molecules SO₂
Explanation:
The Person is right
into both ends of a meter-long glass tube samples of gases are introduced simultaneously. one end receives hydrogen chloride gas (hcl) while the other end receives ammonia gas (nh3). when the gases meet in the tube, they react to form solid ammonium chloride (nh4cl). where in the tube does the nh4cl form?
Answers
Option (B) closer to the end where HCl was introduced is correct because, according to Graham's Law of Diffusion, The Rate of diffusion is indirectly proportional to 1/√(Molar. mass).
The ammonium chloride (NH₄Cl) will form primarily in the middle of the glass tube, where the two gases (NH₃ and HCl) are first able to react with one another. This is because the gases travel through the tube at different velocities, so the reaction will occur where the gases first meet.
As the reaction progresses, the ammonium chloride will continue to form until the reaction is complete and the tube is filled with the solid product.
The reaction of the two gases is reversible, meaning that the NH₄Cl can also break down back into NH₃ and HCl. However, as the gases continue to travel through the tube, the reaction rate of the NH₄Cl breaking down is much slower than the rate at which it is formed.
Therefore, the majority of the ammonium chloride will form in the middle of the tube where HCl is introduced.
To know more about Graham's Law, click below:
https://brainly.com/question/12099514
#SPJ4
What are some potential traits that are needed to become a super athlete?
Answers
Answer: Confidence, strength, potential
Explanation:
\( \huge \boxed{ \fcolorbox{black}{pink}{Answer}}\)
20 Distinguishing Personality Traits of High-Performing Athletes
1. Self Confidence. “Self-Confidence” isn't just a phrase for cheesy motivational posters. ...
2. Strong Sense of Motivation. It takes more than a shiny medal or hefty check to motivate the world's best athletes. ...
3. Inner Desire to Succeed. ...
4. Natural Goal Setter. ...
5. Self-Discipline. ...
6. Optimism. ...
7. Sense of Belonging. ...
8. Natural Leader.
Which activity would be considered resource extraction?
A) eating beef
B) raising cattle
C) hunting deer
D) eating deer
Answers
Answer:
Answer: hunting deer
Explanation:
since the extraction of resources to the withdrawing of materials from the enviroment for human use, including fossil fuels ( oil, gas, and coal), rocks and minerals, bimass via deforestation and fishing and hunting, and water.
100 cm³ of a gas at 27°C is cooled to 20°C at constant pressure .Calculate the volume of gas at 20°C.
Answers
According to Charle's law, the volume of the given mass of a gas is directly proportional to its absolute temperature provided that the pressure is constant. Mathemically;
\(\begin{gathered} V\alpha T \\ V=kT \\ k=\frac{V}{T} \\ k=\frac{V_1}{T_1}=\frac{V_2}{T_2} \end{gathered}\)where;
V1 and V2 are the initial and final volume of the gas
T1 and T2 are the initial and final temperatures of the gas (in Kelvin)
Given the following parameters:
\(\begin{gathered} V_1=100\operatorname{cm}^3 \\ T_1=27^0C=27+273=300K \\ T_2=20^0C=20+273=293K \\ V_2=\text{?} \end{gathered}\)Substitute the given parameters into the formula;
\(\begin{gathered} V_2=\frac{V_1T_2}{T_1}^{} \\ V_2=\frac{100\times293}{300} \\ V_2=\frac{29300}{300} \\ V_2=\frac{293}{3} \\ V_2=97.67\operatorname{cm}^3 \end{gathered}\)Therefore the volume of the gas at 20°C is approximately 97.67cm³
A typical airbag in a car is 139 liters. How many grams of sodium azide needs to be loaded into an airbag to fully inflate it at standard temperature and pressure?
Answers
Approximately 0.268 grams of sodium azide needs to be loaded into the airbag to fully inflate it at standard temperature and pressure.
To calculate the amount of sodium azide required to inflate an airbag, we first need to understand the chemical reaction that takes place. The sodium azide reacts with the potassium nitrate inside the airbag to produce nitrogen gas, which inflates the bag. The reaction is as follows:
\(2NaN_3 + 2KNO_3 \rightarrow3N_2 + 2Na_2O + K_2O\)
From the balanced chemical equation, we can see that 2 moles of sodium azide (NaN3) react to produce 3 moles of nitrogen gas (N2).
The volume of the airbag is given as 139 liters, which is equivalent to 0.139 cubic meters. At standard temperature and pressure (STP), the volume of one mole of gas is 22.4 liters. Therefore, the number of moles of nitrogen gas required to fill the airbag is:
n = V/STP = 0.139/22.4 = 0.00620 moles
To produce 3 moles of nitrogen gas, we need 2 moles of sodium azide. Therefore, the number of moles of sodium azide required is:
n(NaAzide) = (2/3) x n(N2) = (2/3) x 0.00620 = 0.00413 moles
The molar mass of sodium azide is 65 grams/mole. Therefore, the mass of sodium azide required to inflate the airbag is:
Mass = n(NaAzide) x Molar mass = 0.00413 x 65 = 0.268 grams
For more such questions on sodium azide
https://brainly.com/question/28379904
#SPJ11
To fully inflate an airbag, about 50 grams of sodium azide is required. This chemical is stored in the airbag and when the sensor detects a crash, it is ignited, producing nitrogen gas which inflates the bag.
Sodium azide is a highly toxic and explosive substance, and must be handled with great care during the manufacturing and installation of airbags. Once the airbag is deployed, the nitrogen gas produced by the reaction of sodium azide with a metal oxide is harmless and rapidly dissipates into the atmosphere.It is important to note that tampering with an airbag or attempting to remove sodium azide from an airbag is extremely dangerous and should never be attempted. Only trained professionals should handle airbag installation and removal.
Learn more about sodium here:
brainly.com/question/28379904
#SPJ11
Medicine. A pharmaceutical company conducts an experiment in which a subject takes 100mg of a substance orally. The researchers measure how many minutes it takes for half of the substance to exit the bloodstream. What kind of variable is the company studying?
Answers
The researchers may use statistical analysis to estimate the half-life and evaluate the substance's pharmacokinetic properties, such as absorption, distribution, metabolism, and elimination.
The variable that the pharmaceutical company is studying in this experiment is a pharmacokinetic variable known as the "half-life" of the substance. The half-life represents the time it takes for the concentration or amount of a substance in the bloodstream to decrease by half.
In this case, the researchers are administering 100mg of the substance orally to the subject and then measuring the time it takes for half of the substance to be eliminated from the bloodstream. The half-life is a crucial parameter in pharmacokinetics as it provides information about the rate of elimination or clearance of the substance from the body.
The half-life variable is a quantitative variable because it represents a measurable quantity, specifically the time duration. It is a continuous variable as it can take any positive value on the time scale, depending on the specific substance being studied. The researchers may use statistical analysis to estimate the half-life and evaluate the substance's pharmacokinetic properties, such as absorption, distribution, metabolism, and elimination.
Learn more about pharmacokinetic from below link
https://brainly.com/question/13355142
#SPJ11
I need help writing about transformation of energy but it has to have a title,objective,hypothesis, procedures and data analysis and conclusion
Answers
Answer:
Title: Investigating the Transformation of Energy
Objective: The objective of this experiment is to observe and analyze the transformation of energy between different forms, such as kinetic, potential, thermal, and electrical energy.
Hypothesis: It is hypothesized that energy can be transformed from one form to another, but the total amount of energy in a closed system remains constant (the law of conservation of energy).
Procedures:
Gather materials, including a pendulum, stopwatch, ruler, and potential energy toy.Set up the pendulum and measure its length, mass, and amplitude.Start the pendulum and measure its period and velocity.Use the potential energy toy to measure the potential energy stored in the toy.Use the stopwatch to measure the time it takes for the potential energy toy to hit the ground.Use a thermometer to measure the temperature of water before and after heating it with an electric heater.Measure the voltage and current of the electric heater and calculate the power used.Measure the distance traveled and time taken by a toy car moving down a ramp, and calculate its average speed.Use a multimeter to measure the voltage and current of a battery, and calculate its electrical energy.Data Analysis:
Analyze the data collected from the pendulum experiment to calculate its kinetic and potential energy, and compare the total energy to the initial potential energy of the pendulum.Calculate the gravitational potential energy of the potential energy toy and compare it to the kinetic energy just before it hits the ground.Use the data collected from the electric heater experiment to calculate the energy used to heat the water and compare it to the electrical energy used by the heater.Calculate the kinetic energy of the toy car and compare it to the gravitational potential energy of the ramp.Calculate the electrical energy stored in the battery and compare it to the energy used to power a light bulb.Conclusion:
The results of this experiment support the hypothesis that energy can be transformed from one form to another, but the total amount of energy in a closed system remains constant. This experiment demonstrated the transformation of energy between kinetic, potential, thermal, and electrical forms, and showed how energy can be transferred and used to do work. Understanding the transformation of energy is important in many fields, including physics, engineering, and environmental science.
Which state of matter of water is the most dense ?
A. Gaseous water vapor
B. Liquid water
C. Solid ice
D. Water and ice are equally dense.
Answers
Write a nuclear equation for the beta decay of the following isotopes: Lead-210
Answers
In this question, we need to determine what will be the nuclear equation for the Beta decay of Lead - 210.
Beta decay is a type of radioactive decay in which we will have either a neutron becoming a proton, this will be called Beta-minus decay or a proton becoming a neutron, this will be called a Beta-plus decay
In the case of Pb-210, we will have a Beta-minus decay, and one neutron will become a proton:
210/82 Pb ---> 210/83 Bi + electron
Lead - 210 will become Bismuth - 210
what is the molarity of a solution that was prepared by dissolving 82.0 g of CaCl2 in enough water to make 812 mL of solution
Answers
The molarity of a solution that was prepared by dissolving 82.0 g of calcium chloride in enough water to make 812 mL of solution is 0.91M.
How to calculate molarity?Molarity is the concentration of a substance in solution, expressed as the number moles of solute per litre of solution
The Molarity of a solution can be calculated using the following formula:
Molarity = no of moles ÷ volume
According to this question, a solution was prepared by dissolving 82.0 g of calcium chloride in enough water to make 812 mL of solution. The molarity can be calculated as follows:
no of moles of Calcium chloride = 82.0g ÷ 110.98 g/mol = 0.74 mol
Molarity = 0.74 ÷ 0.812 = 0.91M
Learn more about molarity at: https://brainly.com/question/29884686
#SPJ1
if the equivalence point is reached after 22.0 ml, what is the original concentration of hno3?
Answers
The original concentration of HNO3 is 0.11 M.
The equivalence point is the point in the titration process where the amount of reactant in one solution is chemically equivalent to the amount of reactant in the other solution.
In acid-base titrations, the equivalence point is the point at which the acid and the base are neutralized and the pH is equal to 7.0 (neutral).The given question is related to titration.
To find the original concentration of HNO3, we need to know the volume of NaOH solution added to the acid in the titration process and the molarity of NaOH solution.
Using these data, we can calculate the moles of NaOH used in the titration. Since the reaction between HNO3 and NaOH is a 1:1 ratio, we can find the moles of HNO3 present in the original solution.
From this, we can calculate the original concentration of HNO3.Let’s assume that the molarity of NaOH solution is x M and the volume of NaOH solution added is 22.0 ml.
According to the balanced chemical equation of the reaction between HNO3 and NaOH,1 mole of HNO3 + 1 mole of NaOH → 1 mole of NaNO3 + 1 mole of H2O
Moles of NaOH used in titration = (22.0 ml) (x M) = 22x/1000 moles
Moles of HNO3 present in original solution = moles of NaOH used in titration = 22x/1000 moles
Assuming the initial volume of HNO3 is 25.0 ml, the moles of HNO3 present in the original solution would be calculated as follows:
Molarity (M) = moles/volume (L)Initial moles of HNO3 = M × V = (22x/1000) moles
Moles of HNO3 present in the original solution = (22x/1000) moles - (1/2) × (22x/1000) moles = 11x/1000 moles
Initial volume of HNO3 = moles/M = (11x/1000) moles / (25/1000) L = (11/25) L = 0.44 L = 440 ml
Therefore, the original concentration of HNO3 is 0.11 M.
Learn more about concentration at: https://brainly.com/question/17206790
#SPJ11
A 2.9 kg model rocket accelerates at 15.3 m/s2 with a force of 44 N. Before launch, the model rocket was not moving. After the solid rocket engine ignited, hot gases were pushed out from the rocket engine nozzle and propelled the rocket toward the sky.
Which of Newton’s laws apply in this example? Check all that apply.
Answers
Answer:
newton's firts law
Explanation:
because it states that everybody continue its state in rest unless an external force is appy to change its state from rest to motion.
Answer:
A, B, C
Explanation:
Copper metal displaces silver (I) ion from aqueous solution, producing silver metal and an aqueous solution of copper (II) ion. Write a balanced equation.
Answers
The balanced chemical equation for this reaction can be represented as follows: 2AgNO3 + Cu → Cu(NO3)2 + 2Ag
Copper metal displaces silver (I) ions from an aqueous solution, producing silver metal and an aqueous solution of copper (II) ions. Here, Copper metal (Cu) reacts with silver nitrate (AgNO3) to form copper nitrate (Cu(NO3)2) and silver metal (Ag). The oxidation state of copper increases from 0 in copper metal to +2 in copper nitrate. On the other hand, the oxidation state of silver decreases from +1 in silver nitrate to 0 in silver metal. Therefore, this reaction is an example of a single displacement reaction.
A single displacement reaction, also known as a substitution reaction, is a type of chemical reaction in which one element or compound replaces another element or compound in a compound. During a single displacement reaction, an element that is more reactive replaces a less reactive element in a compound. A general equation for a single displacement reaction is A + BC → AC + B, where A and B are elements or ions, and BC is a compound. The product AC is a new compound formed when A replaces B in compound BC. The reaction is said to be a single displacement reaction because only one element or ion is displaced during the reaction. Copper metal is more reactive than silver (I) ion in an aqueous solution. Therefore, copper metal displaces silver (I) ions from the aqueous solution, producing silver metal and an aqueous solution of copper (II) ions. The balanced chemical equation for this reaction can be represented as 2AgNO3 + Cu → Cu(NO3)2 + 2Ag. In this reaction, copper metal replaces the silver (I) ion in silver nitrate (AgNO3) to form copper nitrate (Cu(NO3)2) and silver metal (Ag).
The copper metal reacts with silver nitrate to form copper nitrate and silver metal. This reaction is an example of a single displacement reaction. During this reaction, copper metal displaces the silver (I) ion from the aqueous solution because it is more reactive than the silver (I) ion. Therefore, this reaction is used to separate copper metal from silver nitrate solution.
To know more about oxidation state visit
brainly.com/question/31688257
#SPJ11
the decomposition of dinitrogen pentoxide is described by the chemical equation 2 n2o5(g) 4 no2(g) o2(g) if the rate of appearance of no2 is equal to 0.560 mol/min at a particular moment, what is the rate of appearance of o2 at that moment?
Answers
If the rate of appearance of NO₂ is 0.560 mol/min at a particular moment, then the rate of appearance of O₂ at that moment is 0.070 mol/min.
The chemical equation for the decomposition of dinitrogen pentoxide is 2 N₂O₅(g) → 4 NO₂(g) + O₂(g).
From this equation, we can see that for every 2 moles of N₂O₅ that decompose, 1 mole of O₂ is produced.
If the rate of appearance of NO₂ is 0.560 mol/min at a particular moment, then we know that the rate of disappearance of
N₂O₅ is 0.560/4 = 0.140 mol/min (since 4 moles of NO₂ are produced for every 2 moles of N₂O₅ that decompose).
Therefore, at that particular moment, the rate of appearance of O₂ is equal to half the rate of disappearance of N₂O₅, which is 0.140/2 = 0.070 mol/min.
This is because the stoichiometry of the reaction tells us that for every 2 moles of N₂O₅ that decompose, 1 mole of O₂ is produced. So, the rate of appearance of O₂ is half the rate of disappearance of N₂O₅.
to know more about decomposition refer here:
https://brainly.com/question/8009068#
#SPJ11
A sample of the Earth’s crust was found to contain over 80% of a material called silicon dioxide. The sample had a volume of 15 cm3 and the mass of the sample was 39. 75 grams.
What is the density of silicon dioxide?
Answers
The density of silicon dioxide is 2.12 g/cm³.
Volume of the sample = 15 cm³
Mass of the sample = 39.75 g
Percent of silicon dioxide = 80%
To find the mass of silicon dioxide present in the sample, we can use the given percentage:
Percent of silicon dioxide / 100 = Mass of silicon dioxide / Mass of the sample
0.8 = Mass of silicon dioxide / 39.75
Mass of silicon dioxide = 31.8 g
Now, let's calculate the density of silicon dioxide:
The formula for calculating density is:
Density = Mass / Volume
We know that:
Mass of silicon dioxide = 31.8 g
Volume of the sample = 15 cm³
Putting the values in the formula:
Density = Mass / Volume
Density = 31.8 g / 15 cm³
Density = 2.12 g/cm³
Therefore, the density of silicon dioxide is 2.12 g/cm³.
Learn more about density
https://brainly.com/question/29775886
#SPJ11
