Answers
The question is incomplete, the complete question is shown in the image attached.
The ion that is not isoelectronic with the noble gas neon is O^2+.
To be "isoelectronic" means to have the same number of electrons. When we say that a specie is isoelectronic with the noble gas neon, we mean that the specie has 10 electrons just as neon does.
All the ions listed in the options have 10 electrons except O^2+ which has six electrons.
Therefore, the ion that is not isoelectronic with the noble gas neon is O^2+.
Learn more: https://brainly.com/question/2094768

Related Questions
Why do you think the mass number
includes only the numbers of protons and neutrons?
Answers
Protons and neutrons make up the majority of an atom's mass because electrons have almost negligible mass. Consequently, an atom's mass in atomic mass units is determined by the sum of its protons and neutrons.
How do the quantity of protons and neutrons affect atomic mass?An element's mass number is determined by the sum of its proton and neutron counts: Protons and neutrons together make up mass. Simply deduct the number of protons, or atomic number, from the mass number to get how many neutrons each atom has.
This becomes significant when there is a mass number. It enables us to distinguish between elements known as isotopes that have differing numbers of neutrons.
Although protons and neutrons are similar in size, they are both significantly heavier than electrons (approximately 2,000 times as massive as an electron). A proton's positive charge is equivalent to an electron's negative charge in strength.
Learn more about protons and neutrons refer
https://brainly.com/question/28476164
#SPJ9
1. In the equation
12 + 5C12 + 6H20-→ 2HIO; + 10HCI
(a) has iodine been oxidized, or has it been reduced?
(b) has chlorine been oxidized, or has it been reduced?
(Figure 17.2)
12.1
Answers
Answer: iodine is oxidised and Chlorine is reduced
Explanation: iodine is oxidised, in I2 oxidation number sim 0,
in HIO oxidation number is +I.
Chlorine is reduced, oxidation number changes from 0 to -I
A 1.0 mole sample of fluorine gas at 25 °C has an average molecular velocity of 415 m/s. What is the total KE of the gas sample? Report your answer in kilojoules to the nearest tenth.
Answers
The total kinetic energy of the gas sample is 3.3 KJ
What is kinetic energy?This is the energy possessed by an object in motion. Mathematically, it can be expressed as:
KE = ½mv²
Where
KE is the kinetic energy m is the mass v is the velocity How to determine the mass of the fluorine gasMolar mass of fluorine gas = 38 g/molMole of fluorine gas = 1 moleMass of fluorine gas = ?Mass = mole × molar mass
Mass of fluorine gas = 1 × 38
Mass of fluorine gas = 38 g
How to determine the KE of the gas sampleMass (m) = 38 g = 38 / 1000 = 0.038 KgVelocity (v) = 415 m/sKinetic energy (KE) =?KE = ½mv²
KE = ½ × 0.038 × 415²
KE = 3272.275 J
Divide by 1000 to express in kilojoule
KE = 3272.275 / 1000
KE = 3.3 KJ
Learn more about energy:
https://brainly.com/question/10703928
#SPJ1
The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 23.5 g of sodium upon decomposition. How much fluorine was formed?
Answers
19.42g of fluorine is produced upon decomposition of sodium fluoride.
What is mass ratio?The mass of a given substance is converted to moles using the molar mass of this substance in the periodic table. Moles of a given substance are then converted to moles of an unknown substance using the molar ratios from the balanced chemical formulas.
Mass ratio is defined as the percentage composition of the masses of elements in a molecule or compound. A compound always has a defined mass fraction of the corresponding element.
Mass ratio of sodium to fluorine = 1.21:1
If the mass of sodium fluoride produced is 23.5 g
Using dimensional Analysis,
(23.5g of sodium/sodium fluoride)×(1 g of Fluorine/1.21 g of sodium)
= 19.42g(g of fluorine/g of sodium fluoride)
Mass of fluorine produced = 19.42g
To know more about mass ratio, visit:
https://brainly.com/question/14561456
#SPJ1
An atom or ion has 41 neutrons, 36 protons, and 36 electrons. Identify the element symbol, and determine the mass number
and charge for this atom or ion.
Answers
Answer:
Element symbol: Kr.
Mass number: 77.
Charge : 0.
Explanation:
Hello,
In this case, since such substance has the same amount of protons and electrons we can infer it is an atom whose number of neutrons is defined by considering its atomic mass or mass number and atomic number which is actually equal to the number of protons and electrons (36):
\(neutrons=atomic\ mass - atomic\ number\)
In such a way, solving for the atomic mass we obtain:
\(atomic\ mass=neutrons+ atomic\ number\\\\atomic\ mass=41+36=77\)
It means that the element is krypton (Kr) as it has 36 electrons and protons so its charge is 0.
Best regards.
Can anyone help please.......
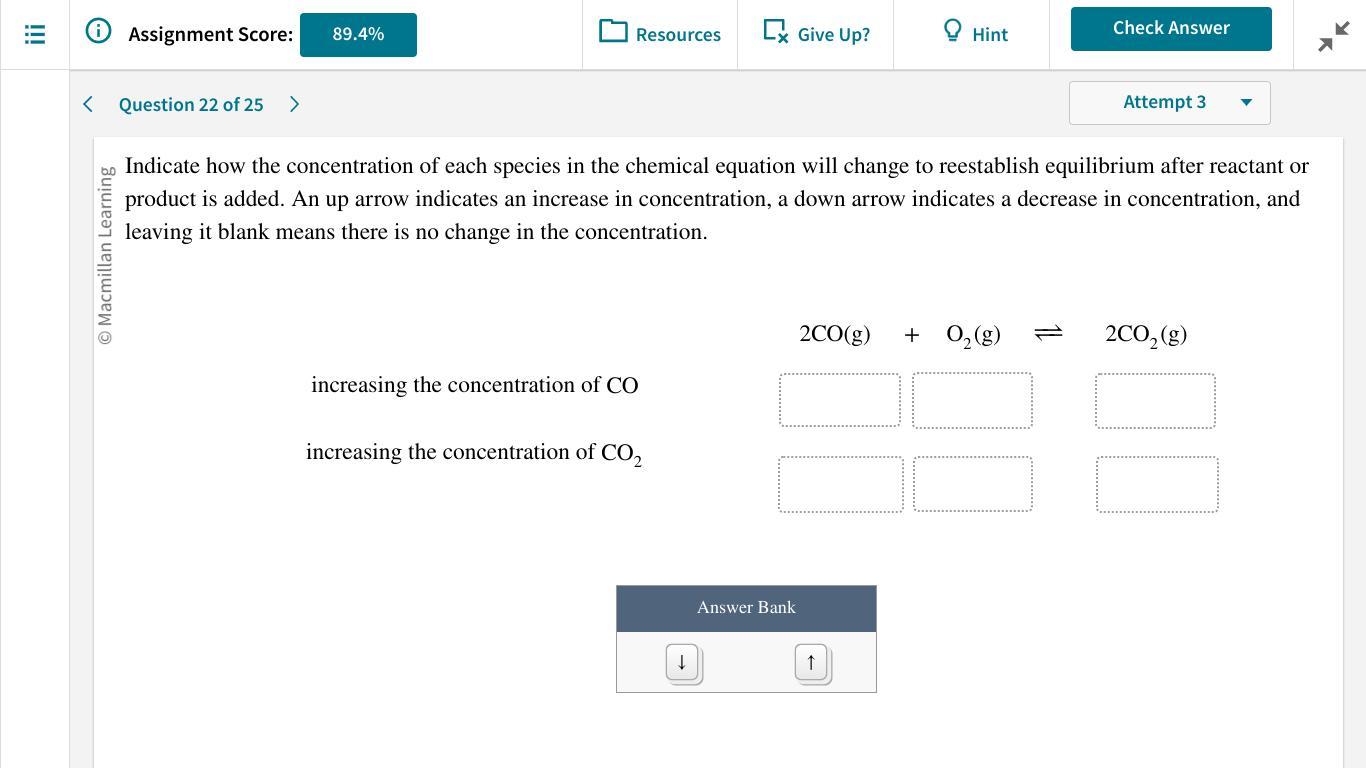
Answers
Increasing the concentration of CO decreases the equilibrium concentration of oxygen and increases the concentration of CO₂, increasing the concentration of CO₂ increases the concentration of CO and O₂.
Chemical equilibrium refers to the state of a system in which the concentration of the reactant and the concentration of the products do not change with time, and the system does not display any further change in properties.
It is the state of a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction. While a reaction is in equilibrium the concentration of the reactants and products are constant.
Learn more about Equilibrium, here:
https://brainly.com/question/30985040
#SPJ1
What is the inertia of motion?
Answers
Answer:
Inertia of motion is the property of a body that opposes any change in it's speed or direction when it is moving.
A Lewis base is any species capable of ________ an electron pair. Select one: a. neutralizing b. donating c. gaining d. accepting e. creating
Answers
Answer:
A Lewis base is any species capable of donating an electron pair.
A transverse wave is shown here. Several wave properties are labeled with letters. Which of these statements accurately reflects the energy associated with the wave? Select ALL that apply. Responses A Y is the frequency, and an increase in Y is associated with an increase in energy.Y is the frequency, and an increase in Y is associated with an increase in energy. B W is the amplitude, and a decrease in W is associated with an increase in energy.W is the amplitude, and a decrease in W is associated with an increase in energy. C W is the amplitude, and an increase in W is associated with an increase in energy.W is the amplitude, and an increase in W is associated with an increase in energy. D Z is the displacement, and a decrease in Z is associated with an increase in energy.Z is the displacement, and a decrease in Z is associated with an increase in energy. E X is wavelength, and a decrease in X is associated with an increase in energy.
Answers
Answer:
C) W is the amplitude, and an increase in W is associated with an increase in energy.
E) X is wavelength, and a decrease in X is associated with an increase in energy.
Explanation:
A transverse wave consists of an oscillating electric field that is perpendicular to the direction of the wave. Increasing either the amplitude or the wavelength of the wave increases its energy. For a transverse wave, the amplitude corresponds to W, while the wavelength is labeled X. So increasing W or decreasing X will create increase the energy of the wave.
Consider the structure of chloride ion. Draw the conjugate acid for chloride ion. Remember to include charges and non-bonding electrons where necessary. Select Draw Rings More Erase H CI C17
Answers
The chloride ion (Cl-) has a single negative charge and a full octet of electrons in its outermost shell. It has a tetrahedral shape, with three equatorial lone pairs and one axial bond to a hydrogen or other positively charged ion.
The conjugate acid of chloride ion is HCl (hydrogen chloride), which is formed when the chloride ion accepts a proton (H+) from an acid. The resulting molecule has a positive charge and a linear shape, with a single bond between the hydrogen and chlorine atoms.
The conjugate acid of chloride ion, HCl, is a strong acid that readily donates a proton (H+) to a base to form the chloride ion. In water, HCl dissociates completely to form H+ and Cl- ions. The hydrogen ion (H+) has a positive charge and no electrons, while the chloride ion (Cl-) retains its original tetrahedral shape and three lone pairs of electrons.
Learn more about electrons here :
https://brainly.com/question/1255220
#SPJ4
How many grams of Cl2 are required to react with 19.5 g of Al?
2AI (s) + 3Cl2 (g) → Al₂Cl6
76.9 g
86.6 g
57.3 g
38.5 g
14.2 g
Answers
The correct answer is 76.9 g of \(Cl_{2}\) is required to react with 19.5 g of \(Al\). Therefore, the correct option is: 76.9 g.
What is Atomic Mass?
Atomic mass, also known as atomic weight or relative atomic mass, is a measure of the mass of an atom of a chemical element. It is expressed in atomic mass units (amu) or unified atomic mass units (u). Atomic mass is a weighted average mass of the naturally occurring isotopes of an element, taking into account their relative abundances.
The balanced chemical equation for the reaction is:
\(2Al\) (s) + 3\(Cl_{2}\) (g) → \(Al_{2}\)\(Cl_{6}\)
From the equation, we can see that the molar ratio between \(Al\)and Cl2 is 2:3. This means that 2 moles of \(Al\) react with 3 moles of \(Cl_{2}\)
To find out how many grams of \(Cl_{2}\) are required to react with 19.5 g of \(Al\) we need to convert the given mass of \(Al\) to moles using its molar mass, and then use the mole ratio from the balanced equation to calculate the amount of \(Cl_{2}\).
The molar mass of \(Al\) is 27 g/mol, and the molar mass of \(Cl_{2}\) is 2 * 35.45 g/mol = 70.9 g/mol.
Moles of \(Al\)= Mass of \(Al\)/ Molar mass of\(Al\)
Moles of \(Al\) = 19.5 g / 27 g/mol ≈ 0.722 mol
According to the mole ratio from the balanced equation, 2 moles of \(Al\)react with 3 moles of \(Cl_{2} .\)
Moles of \(Cl_{2}\)= Moles of \(Al\) * (3 moles of \(Cl_{2}\) / 2 moles of \(Al\))
Moles of \(Cl_{2}\) = 0.722 mol * (3/2) ≈ 1.083 mol
Now, we can convert moles of \(Cl_{2}\) to grams using its molar mass.
Mass of\(Cl_{2}\) = Moles of \(Cl_{2}\) * Molar mass of \(Cl_{2}\)
Mass of\(Cl_{2}\) = 1.083 mol * 70.9 g/mol ≈ 76.9 g
Learn more about Atomic Mass from the given link
https://brainly.com/question/3187640
#SPJ1
Fish in the Antarctic Ocean swim in water at about -2°C. (a) To prevent their blood from freezing, what must be the concentration (in molality) of the blood? Is this a reasonable physiological concentration? (8pts) (b) In recent years, scientists have discovered a special type of protein in the blood of these fish that, although present in quite low concentrations (≤ 0.001 m), has the ability to prevent the blood from freezing. Suggest a mechanism for its action. (2 pts) (total 10 pts)
Answers
This means that the molality of solutes in the blood of fish in the Antarctic Ocean must be at least -1.08 m to prevent it from freezing at -2°C.
What is concentration?Concentration refers to the amount of a solute (the substance being dissolved) that is present in a given amount of a solvent (the substance doing the dissolving). It is typically expressed as the amount of solute per unit volume or mass of the solution. There are several different ways to express concentration, including mass concentration, molar concentration, molality, and mole fraction. Concentration is an important concept in chemistry and is used to describe the strength of solutions, as well as to calculate reaction rates, equilibrium constants, and other properties of chemical systems.
Here,
(a) To prevent the blood of fish in the Antarctic Ocean from freezing, the concentration of solutes (such as salts or proteins) in their blood must be high enough to lower the freezing point of water below -2°C. The molality (moles of solute per kilogram of solvent) of the blood can be calculated using the formula:
ΔTf = Kf * m
where ΔTf is the change in freezing point, Kf is the freezing point depression constant for water (1.86°C/m), and m is the molality of the solution.
ΔTf = -2°C - 0°C = -2°C
Kf = 1.86°C/m
Therefore, m = ΔTf / Kf = -2°C / 1.86°C/m = -1.08 m
This is a very high concentration, but it is a reasonable physiological concentration for these fish as they have adapted to living in such extreme environments.
(b) The special type of protein in the blood of these fish that prevents it from freezing is called an antifreeze protein (AFP). The mechanism by which AFPs prevent ice formation is known as the adsorption inhibition mechanism. AFPs bind to the surface of ice crystals, preventing them from growing and aggregating, and thus inhibiting ice formation. This allows the fish to maintain fluidity of their blood and bodily fluids in subzero temperatures. The exact mechanism of how AFPs adsorb to the ice surface is still being studied, but it is thought to involve specific amino acid residues and hydrogen bonding interactions between the protein and the ice surface.
To know more about concentration,
https://brainly.com/question/29276511
#SPJ9
The rate constant for the reaction below was determined to be 3.241×10-5 s–1 at 800 K. The activation energy of the reaction is 255 kJ/mol. What would be the value of the rate constant at 9.30×102 K?
Answers
The value of the rate constant at 9.30×102 K is 2.03×102 s–1.
Rate constant from Arrhenius equation calculation.
To solve this problem, we can use the Arrhenius equation:
k = A * exp(-Ea/RT)
where:
k = rate constant
A = pre-exponential factor or frequency factor
Ea = activation energy
R = gas constant (8.314 J/mol·K)
T = temperature in Kelvin
We can rearrange this equation to solve for the rate constant at the new temperature:
k2 = A * exp(-Ea/RT2)
where k2 is the rate constant at the new temperature T2.
We are given k1 = 3.241×10-5 s–1 at T1 = 800 K, and Ea = 255 kJ/mol. We need to find k2 at T2 = 9.30×102 K.
To find A, we can rearrange the Arrhenius equation to solve for A:
A = k / exp(-Ea/RT)
Using k1 and T1, we get:
A = 3.241×10-5 s–1 / exp(-255000 J/mol / (8.314 J/mol·K * 800 K))
A = 7.07×1013 s–1
Now we can use A, Ea, k1, and T2 to find k2:
k2 = A * exp(-Ea/RT2)
k2 = 7.07×1013 s–1 * exp(-255000 J/mol / (8.314 J/mol·K * 930 K))
k2 = 2.03×102 s–1
Therefore, the value of the rate constant at 9.30×102 K is 2.03×102 s–1. using Arrhenius equation.
Learn more about Arrhenius equation below.
https://brainly.com/question/14739712
#SPJ1
1. Matter can (change form/be created)
Answers
Answer: Yes
Explanation:
Duh it can change. Because matter is what we are made of, and if we change, then matter also changes along with us :)
How many moles are in 39.5 grams of Lithium?
SHOW HOW YOU SET UP THE CONVERSION
SHOW WHAT MATH OPERATION
INCLUDE THE APPROPRIATE UNITS IN YOUR ANSWER
Answers
Answer:
185.05 g.
Explanation
Firstly, It is considered as a stichiometry problem.
From the balanced equation: 2LiCl → 2Li + Cl₂
It is clear that the stichiometry shows that 2.0 moles of LiCl is decomposed to give 2.0 moles of Li metal and 1.0 moles of Cl₂, which means that the molar ratio of LiCl : Li is (1.0 : 1.0) ratio.
We must convert the grams of Li metal (30.3 g) to moles (n = mass/atomic mass), atomic mass of Li = 6.941 g/mole.
n = (30.3 g) / (6.941 g/mole) = 4.365 moles.
Now, we can get the number of moles of LiCl that is needed to produce 4.365 moles of Li metal.
Using cross multiplication:
2.0 moles of LiCl → 2.0 moles of Li, from the stichiometry of the balanced equation.
??? moles of LiCl → 4.365 moles of Li.
The number of moles of LiCl that will produce 4.365 moles of Li (30.3 g) is (2.0 x 4.365 / 2.0) = 4.365 moles.
Finally, we should convert the number of moles of LiCl into grams (n = mass/molar mass).
Molar mass of LiCl = 42.394 g/mole.
mass = n x molar mass = (4.365 x 42.394) = 185.05 g.
Answer:
5.69
Explanation:
1 gram of lithium is .144 of a mole, since you have 39.5 grams of lithium you can multiply .144 by 39.5 to get an answer of 5.69
I need help right now please

Answers
Answer:
Explanation:
It is A because the electrice flows through the cord to give energy for the blades to move.
A proton in a linear accelerator has a de Broglie wavelength of 154 pm.
▼
Part A
What is the speed of the proton?
Express your answer with the appropriate units.

Answers
So, the speed of the proton is approximately 2.30 x 10⁶ m/s.
What is the concept of de Broglie wavelength?The de Broglie wavelength is a concept in quantum mechanics that was introduced by Louis de Broglie in 1924. It refers to the wavelength associated with a moving particle, such as an electron, proton, or other type of particle, and is proportional to its momentum.
To find the speed of the proton (v): v = h/(mλ)
v = (6.626 x 10⁻³⁴ Js) / [(1.67 x 10⁻²⁷kg)(154 x 10⁻¹² m)]
= 2.30 x 10⁶ m/s
To know more about wavelength visit :-
brainly.com/question/13533093
#SPJ1
Please help I only need this one to finish!

Answers
Answer:
2CO(g) + O2(g) ⇌ 2CO2(g)
Increasing the concentration of CO - ↓ ↓ ↑
Increasing the concentration of CO2 - ↑ ↑ ↓
Explanation:
The given reaction is
2CO(g) + O2(g) ⇌ 2CO2(g)
a) When the concentration of CO is increased, the stress is relieved as the reaction that consumes the added CO occurs more rapidly than its reverse reaction, for example., the forward reaction rate increases. The equilibrium will shift in favor of the product. However, the concentration of reactants (CO and O2) decreases and product concentration (CO2) increases.
b) When the concentration of CO2 is increased, the stress is relieved as the reaction that consumes the added CO2 occurs more rapidly than its reverse reaction, for example., the rate of backward reaction increases. The equilibrium will shift in favor of the reactant. Therefore, the concentration of reactants (CO and O2) increases, and the product concentration (CO2) decreases.
Hope this helps!
How many electron shells/orbitals will be around the nucleus of an atom of aluminum?
A. 1
B. 2
C. 3
4. 4
Answers
The number of electrons is revealed by the atomic number. This indicates that an aluminum atom contains 13 electrons. Eight electrons make up shell 1, eight electrons make up shell 2, and three electrons make up shell 3. The correct option is C.
Each subsequent shell that surrounds the nucleus of an atom's electrons is placed farther away from the nucleus. Atomic orbitals make up one or more of the one or more subshells that make up an electron shell.
The first two electrons in aluminum fall into the 1s orbital, followed by the next two electrons into the 2s orbital. The following six electrons complete the second shell's 2p orbital. The 3s orbital is then filled by electrons 11 and 12. The 3p orbital is now occupied by the final electron.
Thus the correct option is C.
To know more about electron shells, visit;
https://brainly.com/question/30464976
#SPJ6
ASAP PLEASE!
The speed of light is 2.998 m/s. What is the speed of light in km/hr.
Answers
Answer:
10.793 kilometres per hour
hey please help man...
1. Cations are ____________ charged and have less ____________than ____________.
2. Anions are ____________ charged and have more ____________ than ____________.
3. Columns on the periodic table are known as ____________.
4. The elements in the first group are called ____________ and have ____________
valence electron(s).
Answers
Answer:
1. Cations are positively charged and have less electrons than protons.
2. Anions are negatively charged and have more electrons than protons.
3. Columns on the periodic table are known as groups.
4. The elements in the first group are called alkalis metals and have one valence electron.
Explanation:
How many moles of HNO3 will be produced
from the reaction of 46.5 g of NO2 with excess
water in the following chemical reaction?
3 NO₂(g) + H₂O (1)→ 2 HNO3(g) + NO(g)
Answers
Answer:
0.674 moles HNO₃
Explanation:
To find the moles of HNO₃, you need to (1) convert grams NO₂ to moles NO₂ (via molar mass) and then (2) convert moles NO₂ to moles HNO₃ (via mole-to-mole ratio from equation coefficients). It is important to arrange the conversions in a way that allows for the cancellation of units. The final answer should have 3 sig figs to match the sig figs of the given value (46.5 g).
Molar Mass (NO₂): 14.007 g/mol + 2(15.998 g/mol)
Molar Mass (NO₂): 46.003 g/mol
3 NO₂(g) + H₂O (l) ------> 2 HNO₃(g) + NO(g)
46.5 g NO₂ 1 mole 2 moles HNO₃
------------------- x ------------------- x -------------------------- = 0.674 moles HNO₃
46.003 g 3 moles NO₂
What is the heat released when 25g water is condensed at 100 C?
Answers
1017.5 Joules is the heat released when 25g water is condensed at 100 C.
When water is condensed, it changes from a gas to a liquid, releasing heat in the process. The amount of heat released can be calculated using the formula:
q = m * ΔH
where:
q = heat released (in Joules)
m = mass of water (in grams)
ΔH = heat of vaporization of water (in J/g)
The heat of vaporization of water at 100°C is 40.7 kJ/mol, or 40.7 J/g. Therefore, substituting the values in the formula, we get:
q = 25 g * 40.7 J/g
q = 1017.5 J
So the heat released when 25 g of water is condensed at 100°C is approximately 1017.5 Joules.
To learn more about vaporization problem at
https://brainly.com/question/14578189
https://brainly.com/question/26127294
An equilibrium mixture of N2, 02, and NO gases at 1500 K is determined to consist of
6.4 x101-3 mol/1 oF N2, 1.7 x 101-3 mol/ of 02 , and 1.1 × 10 ^-5 mol/1 of NO. What is the equilibrium constant for the system at this temperature?
Answers
The equilibrium constant for the system at this temperature is\(1.17 × 10^-31 mol^2/L^2\).
For the chemical equation:
N2(g) + O2(g) ⇌ 2NO(g)
The equilibrium mixture at a temperature of 1500 K is determined to contain 6.4 × 10^-3 mol/L of N2,\(1.7 × 10^-3\)mol/L of O2 and 1.1 × 10^-5 mol/L of NO. First, we need to calculate the concentration of N2 and O2 required to produce
1.1 × 10^-5 mol/L of NO:
2NO(g) = N2(g) + O2(g)
Given that there are 1.1 × 10^-5 mol/L of NO, the number of moles of N2 and O2 are equal since the stoichiometric ratio is 1:1. Therefore:
\(1.1 × 10^-5 mol/L\) = [N2][O2]Kc = \(([NO]^2)/([N2][O2])Kc\)= \((1.1 × 10^-5 mol/L)^2/(6.4 × 10^-3 mol/L)(1.7 × 10^-3 mol/L)Kc\) =
1.17 × 10^-31 mol^2/L^2.
for such more questions on equilibrium
https://brainly.com/question/5081082
#SPJ8
How many Oxygen atoms are in 12C02
Answers
Answer:
Two oxygen atoms and one carbon atom.
How does the state of matter affect the behavior of molecules?
Answers
Answer:
The behavior of molecules in different phases of matter represents a balance between the kinetic energies of the molecules and the attractive forces between them. All molecules are attracted to each other. The molecules are in the solid-state. At higher temperatures, the kinetic energy of the molecules is higher.
Molecules are the various elements linked together and are present in the matter. The molecules in different phases of matter are represented by kinetic energies and attractive forces.
What is the kinetic molecular theory of matter?The kinetic molecular theory of matter elucidates the composition of the matter of particles of tiny size. The theory explains matter like gas, liquids, and solids.
The different states of matter explain the constant movement and negligible intermolecular force and volume of the particles. The particle matter is said to undergo elastic collision.
The molecules of solids have low kinetic energy whereas the molecules of liquids and gases show high kinetic energy as the particles are placed at larger distances.
Therefore, the state of matter affects the behavior of molecules.
Learn more about kinetic molecular theory here:
https://brainly.com/question/15013597
#SPJ6
How much water would I need to add to 700 mL of a 2.7 M KCl solution to make a 1.0 M solution?
Answers
Answer:
\(1190\ \text{mL}\)
Explanation:
\(M_1\) = Initial Concentration of KCl = 2.7 M
\(V_1\) = Volume of KCl = 1 M
\(M_2\) = Final concentration of KCl = 1 M
\(V_2\) = Amount of water
We have the relation
\(M_1V_1=M_2V_2\\\Rightarrow V_2=\dfrac{M_1V_1}{M_2}\\\Rightarrow V_2=\dfrac{2.7\times 700}{1}\\\Rightarrow V_2=1890\ \text{mL}\)
The amount of water that is to be added is \(1890-700=1190\ \text{mL}\).
Which of the following would be defined as an exact number?
A. bronze is 12.5% by mass tin
B. 25 ml
C. 50.00 cm
D. 1.000 liters
Answers
The value of AG at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,
25 (s, rhombic) + 302 (g) → 2SO3 (g)
AG-370.4 kJ/mol.
+740.0
-740.8
-200,
kJ/mol.
+200.
Answers
The value of ΔG at 25 °C for the given reaction is: ΔG = -370.4 kJ/mol + 0 = -370.4 kJ/mol So, the correct answer is -370.4 kJ/mol
To determine the value of ΔG (Gibbs free energy) at 25 °C for the given reaction:
25 (s, rhombic) + 3/2 \(O_2\)(g) → \(2SO_3\)(g)
We can use the equation:
ΔG = ΔG° + RT ln(Q)
where:
ΔG is the standard Gibbs free energy change
ΔG° is the standard Gibbs free energy change under standard conditions
R is the gas constant (8.314 J/(mol·K) or 0.008314 kJ/(mol·K))
T is the temperature in Kelvin (25 °C = 298 K)
Q is the reaction quotient, which is the ratio of the concentrations of the products to the concentrations of the reactants at a given point during the reaction.
Given that ΔG° is -370.4 kJ/mol, we can plug the values into the equation:
ΔG = -370.4 kJ/mol + (0.008314 kJ/(mol·K) * 298 K) * ln(Q)
Now, we need to determine the value of Q. Since all reactants and products are in their standard states, Q = 1, as their concentrations are taken to be 1.
ΔG = -370.4 kJ/mol + (0.008314 kJ/(mol·K) * 298 K) * ln(1)
Since ln(1) = 0, the term (0.008314 kJ/(mol·K) * 298 K) * ln(1) becomes 0.
Therefore, the value of ΔG at 25 °C for the given reaction is:
ΔG = -370.4 kJ/mol + 0 = -370.4 kJ/mol
So, the correct answer is -370.4 kJ/mol.
For more such questions on reaction visit:
https://brainly.com/question/11231920
#SPj8
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8