what is the ph of a 0.045 m strontium hydroxide
Answers
The pH of the 0.045 M strontium hydroxide solution is approximately 12.95.
The pH of a 0.045 M strontium hydroxide (Sr(OH)2) solution can be calculated using the formula pH = -log10[H+].
However, since Sr(OH)2 is a strong base, we first need to determine the hydroxide ion (OH-) concentration.
Strontium hydroxide dissociates into one Sr2+ ion and two OH- ions.
Therefore, the concentration of OH- ions in the solution is 2 x 0.045 M = 0.09 M.
Now we can calculate the pOH, which is the negative logarithm of the hydroxide ion concentration:
pOH = -log10[OH-]
= -log10(0.09)
≈ 1.05.
To find the pH, we can use the relationship pH + pOH = 14.
So, pH = 14 - pOH
= 14 - 1.05
≈ 12.95.
To know more about the pH of a solution, click below.
https://brainly.com/question/15463092
#SPJ11
Related Questions
Large sections of troposphere with same temperature and humidityA. Air massB. Cold frontC. Stationary frontD. Hurricane
Answers
A large body of air with generally uniform temperature and humidity is referred to as an air mass.
The motion of an air mass is usually determined by the air flow in the upper atmosphere. The motion and strength of air masses are affected by changes in the intensity and position of the jet stream. Air masses converge to form boundaries known as "fronts" where they meet.
The movement of air masses also influences where much of the precipitation falls. Cold air masses have denser air than warmer air masses. As a result, as these cold air masses move, the dense air undercuts the warmer air masses, forcing the warmer air to rise above the colder air.
To know more about Air mass go through:-
https://brainly.com/question/18201898
#SPJ4
The weak ionization constant (Ka)for HCO3 is equal to:AB[H3O+][CO32- ](HCO3-)[HCO3 -[H3O+][CO32-]
![The weak ionization constant (Ka)for HCO3 is equal to:AB[H3O+][CO32- ](HCO3-)[HCO3 -[H3O+][CO32-]](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/fFdZ9Lmdw8Dd0qIkJOMmwVFihe6UpcQX.jpeg)
Answers
Answer
A
\(\frac{\lbrack H_3O^+\rbrack)(\lbrack C(O_3)^2\rbrack}{\lbrack HCO_3^-\rbrack}\)Explanation
The ionization of HCO₃⁻ in H₂O is:
\(HCO₃⁻+H₂O\rightleftarrows H_3O^++CO₃^{2-}\)The general ionization constant, Ka is given as:
\(Ka=\frac{\lbrack Products\rbrack}{\lbrack Reactants\rbrack}\)Hence, the ionization (Ka) of HCO₃⁻ is equal to:
\(\frac{\lbrack H_3O^+\rbrack\lbrack CO_3^{2-}\rbrack}{\lbrack HCO_3^-\rbrack}\)Option A is the correct answer.
How many grams are in 3.13 X 10^26 molecules of O₂?
Help pleaseee
Answers
3.13 x 10^26 molecules O2 x (32.00 grams/6.02 x 10^23 molecules) = 0.0524 grams O2
The first location of chemical and mechanical digestion is the stomach. true/ false
Answers
The first location of chemical and mechanical digestion is the stomach. This statement is false.
Digestion is the breaking down of large insoluble food molecules into smaller water-soluble food molecules so that they can be absorbed into the aqueous plasma. In certain organisms, these small substances are absorbed from the small intestine into the bloodstream. Digestion is important for breaking down food into nutrients that the body uses for energy, growth, and cell repair that it needs to survive.
The digestive system has many forms. A basic distinction is made between internal and external digestion. External digestion evolved early in evolutionary history, and most fungi still rely on it. During this process, enzymes are secreted into the organism's environment, where organic substances are decomposed and some of the products diffuse back into the organism. Animals have a tube (alimentary canal) where internal digestion takes place. This is more efficient as more decomposition products can be trapped and the internal chemical environment can be more effectively controlled.
Learn more about digestion here
https://brainly.in/question/346470
#SPJ4
Help me i don’t know what to put here pls thank you!

Answers
What substance converts the inactive pepsinogen to its active form, pepsin? a. amino acid b. glycine c. hydrochloric acid d. amylase.
Answers
The substance that converts the inactive pepsinogen to its active form, pepsin, is hydrochloric acid.
Pepsinogen is the inactive precursor of pepsin, an enzyme involved in protein digestion.
Pepsinogen is produced and secreted by the chief cells in the stomach. However, it is initially inactive to prevent self-digestion of the stomach lining.
When food enters the stomach, parietal cells in the gastric glands secrete hydrochloric acid (HCl). Hydrochloric acid creates an acidic environment in the stomach, which is necessary for the activation of pepsinogen.
The low pH of the stomach acid causes the denaturation and unfolding of pepsinogen, resulting in its conversion to pepsin.
Pepsin, in its active form, plays a crucial role in breaking down proteins into smaller peptides during the process of digestion. It is particularly effective in cleaving peptide bonds adjacent to certain amino acids, such as phenylalanine and tyrosine.
In summary, hydrochloric acid is responsible for converting the inactive pepsinogen into its active form, pepsin, by providing the acidic environment necessary for the enzymatic activation in the stomach.
Learn more about pepsin from the given link
https://brainly.com/question/15604041
#SPJ11
which diagram represents an electrolytic solution
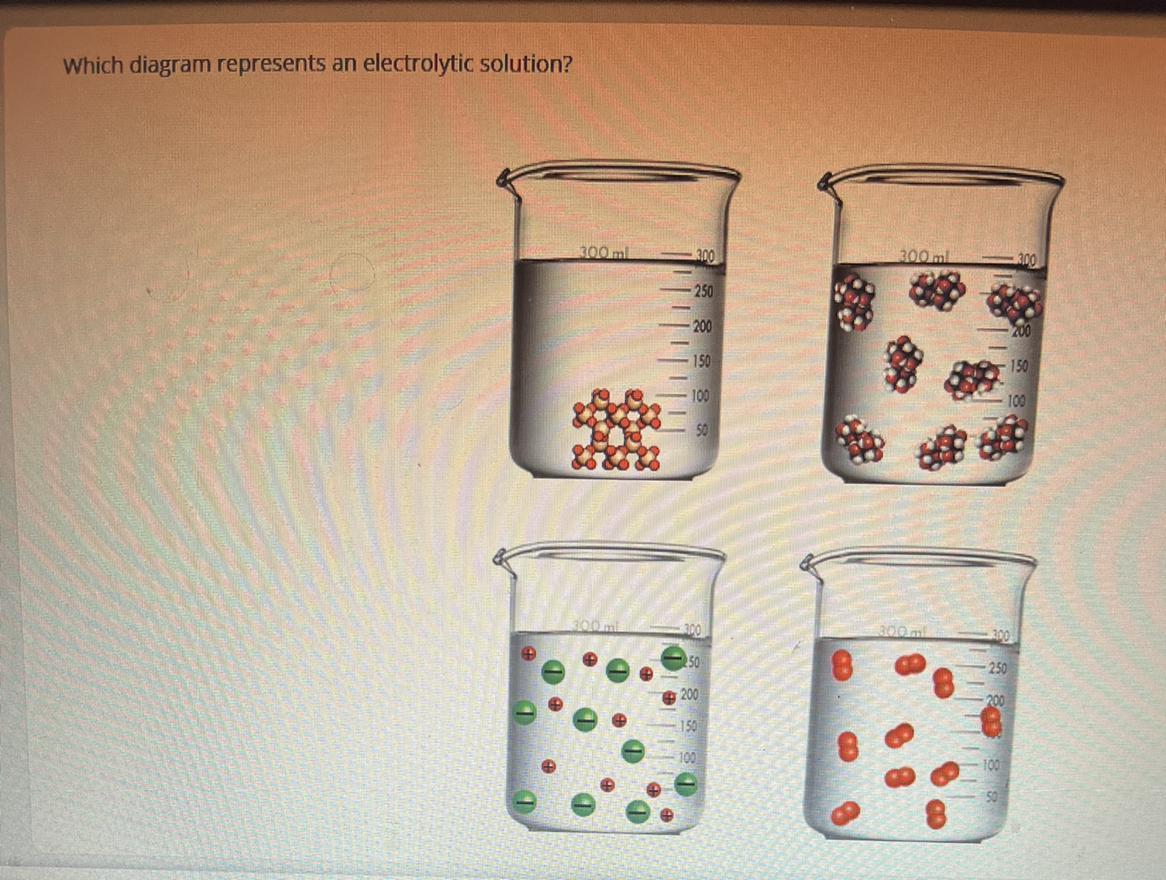
Answers
Answer:
Image
Explanation:
An electrolytic solution is a solution that has the ability of conducting electricity. Electrolytic solutions contain ions in which it conducts electricity from those ions. Ions are charged atoms. This solution (refer to image) has ions, as represented by the + and - symbols.

f there is more than one possible site in the molecule/ion, focus on the central or the charged atom. a) b) c)
Answers
Answer:
This statement is in the context of Lewis Structures, which are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. The diagrams show the valence electrons of the atoms that participate in a bond.
When there is more than one possible site in the molecule or ion, the central or the charged atom must be focused on.
To determine which atom is the central or charged atom, you should look at the molecular/ionic structure and identify the atom with the highest electronegativity or charge density.
In general, the atom with the highest electronegativity or charge density is the central or charged atom.
Learn more about electronegativity here:
https://brainly.com/question/17762711#
#SPJ11
Describe the general trend in electronegativity as the metals in Group 2 on the Periodic Table are considered in order of increasing atomic number.
Answers
Answer:
It decreases down the group
Explanation:
In group 2 of the periodic table, the value of the electronegativity decreases as we go down the group.
Electronegativity of an element is a property that combines the ability of its atom to lose and gain electrons.
It is a measure of the relative tendency with which atoms of the element attracts valence electrons.
Across a period, electronegativity increases from left to right. Down a group, it decreases.In group 2, the electronegativity decreases downward.
Electronegativity is the ability of an atom to attract the electrons to form a covalent.
The general trend of electronegativity increases across the periods and groups.
The trend of electronegativity of Group 2 can be explained as:
1. In group 2, the electronegativity varies with the increase in the atomic number of the elements. The electronegativity decreases as the atomic number increases.
2. The electronegativity increases because the outer electrons are shielded due to the strong attraction of the nucleus.
3. The highest electronegative element of Group 2 is Be, and the least is Barium.
Thus, the electronegativity decreases down the group with an increase in the atomic number.
To know more about electronegativity, refer to the following link:
https://brainly.com/question/2060520
what is saturated solution?12
Answers
Answer:
A solution in which the maximum amount of solvent has been dissolved
Explanation:
For example, 36g of salt in 100g of water.
If element X has 5 valence electrons, what would you expect it to do to fulfil its octet? pls help

Answers
Answer:
a
Explanation:
Answer:
Gain 3 electrons.
Explanation:
If element X have 5 valence electrons it will more than likely gain 3 electrons to fill its octet to become stable. Gaining the 3 electrons is much easier for the atom than if it were to give away it's 5 valence electrons. Elements with 5 valence electrons that could represent element X are:
NitrogenPhosphorusArsenicAntimonywhy is hydrogen not used as fuel for domestic purposes
Answers
Explanation:
It is because hydrogen is a highly combustible and it reacts explosively when it comes in contact with air. And hence as a result, storing of the hydrogen gas is difficult and is dangerous at the same time.
Please help!! I don’t know what to do.

Answers
Answer:
C
Explanation:
No reaction because not enough moles of silver are present for the reaction to occur.
What is the main function of this organ system It protects vital organs from injury. It is the first line of defense and keeps the body warm. It transports nutrients and oxygen to body cells. It produces hormones that regulates growth and metabolism.
Answers
Answer:
Organ systems & their main functions
Explanation:
The chest bones Rib Cage & spine - protects the vital organs (heart, lung,liver) from injury & provides structural support for body.
Skin is the body's first line of defense & keeps the body warm. In case of any invading infection, fever - body temperature rise fights it.
Circulatory system (containing heart) & its body fluid blood - transports nutrients and oxygen to body cells
Endocrine system regulates growth, produces hormones - that target organs via bloodstream.
Determine the volume (mL) required to prepare each of the following. 0.250 L of a 0.175 M KCl solution using an 8.15 M KCl solution.
Answers
Explanation:
When working with dillutions we can use this formula:
Vc * Mc = Vd * Md
Vc = Vd * Md/Mc
Where Vc and Mc are the volume and molarity of the concentrated solution and Vd and Md are the volume and molarity of the dilluted solution. Using that formula we can find the volume of the concentrated solution that we will have to mix with water to get the dilluted solution.
Vc = ? Mc = 8.15 M Vd = 0.250 L Md = 0.175 M
Vc = Vd * Md/Mc
Vc = 0.250 L * 0.175 M/(8.15 M)
Vc = 0.00537 L
1000 mL = 1 L
Vc = 0.00537 L * 1000 mL/(1 L)
Vc = 5.37 mL
Answer: We have to take 5.37 mL from the concentrated solution.
What is a resistor?
1.A battery
2.A light bulb
3.A switch
Answers
Answer:2
Explanation:
2. What type of compound is water (H20) and why is it called a universal solvent?
Answers
Answer:
H2O is a covalent compound and is called a universal solvent because it dissolves most substances than any other known liquid
The universal solvent, H₂O, is a covalent molecule that dissolves more compounds than any other liquid.
Briefing:Water makes an excellent solvent because of its physical and chemical composition. In water molecules, hydrogen and oxygen atoms are organized polarly, with hydrogen possessing a positive electric charges and oxide having a negative electrical charge.
Why water is called universal solvent ?Water is referred to be the "universal solvent" because it has a wider range of dissolving abilities than any other liquid. Every living thing on the world requires this. It indicates that wherever water flows, whether through the air, the ground, or our bodies, it carries valuable molecules, minerals, and nutrients.
To know more about Universal solvent visit:
https://brainly.com/question/2166468
#SPJ2
the trunk of a certain tree is 50 cm thick. Each year it gets thicker by 1 cm. How thick will the tree trunk be in 50 years
Answers
Starting thickness was 50 cm
1 cm every 50 years= 1x50
1x50=50
Old thickness + New thickness= 50 + 50 = 100 cm
:)
Helpppppppp I honestly have no clue

Answers
What are the hydronium and hydroxide ion concentrations in a solution whose ph is 6.52?
Answers
A solution with a pH of 6.52 has a hydronium ion concentration of 3.02x10-7 mol/L and a hydroxide ion concentration of 3.31x10-8 mol/L.
The hydronium ion concentration of a solution can be calculated from pH by using \(10^{-pH}\). For a pH of 6.52, hydronium ion concentration is 3.02x10-7 mol/L.
The concentration of hydroxide ions can be determined by identifying the value of pOH. The sum of pOH and pH is equal to 14, which is based on the negative logarithm of the ion-product constant of water. At a pH of 6.52, pOH is equal to 7.48.
The relationship between pOH and hydroxide ion concentration is the same as the relationship between pH and hydronium ion concentration. With this, the hydroxide ion concentration at pOH of 7.48 is \(10^{-7.48}\) or 3.31x10-8 mol/L.
For more information regarding pH and pOH, please refer to the link https://brainly.com/question/13557815.
#SPJ4
BRAINLY to whoever can answer this simply
Based on your knowledge of chemistry, explain what the reactants and the products of the chemical reaction are.
Thank you :)

Answers
Answer: Reactants are starting materials and are written on the left-hand side of the equation. Products are the end result of the reaction and are written on the right-hand side of the equation.
Explanation:
Hope this helps!! And you're welcome!
How does oxygen obey the octet rule
Answers
Answer:
oxygen obey the octet rule when reacting to form compounds by adding 2 electrons.
Explanation:
Answer:
See below.
Explanation:
An oxygen atom, in free state has 8 electrons, 2 in the first shell, and 6 in the second. It can only achieve stability if the valency of the valence shell is 0. In order for this, it combines with another atom with the same valency, such as magnesium or another oxygen atom to obey the octet rule. Hope it helps.
a gas has a volume of 7L and a mass of 4.44*10^5 micrograms. what is it's density
Answers
Answer:
Explanation:
D = mass/volume.
for the units, I'll take it in g/L but I don't know which units you want.
4.44*10^5 mg = 0.444 grams.
0.444/7 = 0.0634 g/L
what do covalent compounds consist of
Answers
Covalent compounds consist of atoms that are held together by covalent bonds. These bonds form when atoms share electrons in order to achieve a stable electron configuration.
Non-metal atoms form covalent compounds, and these atoms have a tendency to gain electrons in order to achieve a stable configuration. They share electrons to form a covalent bond. The electrons shared in a covalent bond are not transferred from one atom to another; instead, they are shared by both atoms. This shared electron pair is called a bonding pair.
In covalent compounds, the number of electrons shared between atoms is known as the coordination number. The coordination number can vary from one to six, depending on the type of covalent compound.
Covalent compounds can be further classified into polar and nonpolar covalent compounds. In polar covalent compounds, the electrons are shared unevenly between the atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other. This creates a dipole moment, meaning the compound has a positive and negative end.
On the other hand, in nonpolar covalent compounds, the electrons are shared equally between the atoms, resulting in a neutral compound with no partial charges.
Examples of covalent compounds include water (H2O), methane (CH4), and carbon dioxide (CO2). These compounds are present in various forms in our everyday lives, from the water we drink to the air we breathe.
Know more about covalent bonds: https://brainly.com/question/3447218
#SPJ11
10. How many moles of CuCl, are in 150 L of a 2.33 M Cuci, solution?
Answers
Answer:
349.5 moles
Explanation:
(I am assuming that there is a typo at the end, where Cuci solution = CuCl solution)
The formula for molarity is:
Molarity = moles in solution / volume of solution
Here, we are given both the Molarity and the volume. So we can plug it into the equation above and get:
2.33 = moles / 150
Now we solve for the moles by doing:
moles = 2.33 * 150
This gives us the answer of 349.5 moles.
chemistry student needs to create a solution with a high concentration of aqueous silver ions for an experiment. Which solid will be most soluble in water and will most easily dissociate into ions?
Answers
The substance that dissolves most readily in water and readily separates into silver ions is silver nitrate.
Thus, silver nitrate is so easily dissolved in water to create an aqueous solution, it is extremely soluble in water. The compound separates into silver ions and nitrate ions during dissolution.
Effective dissociation in water is caused by the strong ionic contact between the silver cation and nitrate anion. As a result, silver nitrate is the best option for preparing a solution with a high concentration of silver ions for the experiment since it offers a high concentration of aqueous silver ions.
Learn more about the silver nitrate here:
https://brainly.com/question/5764610
#SPJ1
If an atom is neutrally charged the number of electrons equals
Answers
Answer:
The number of protons
Explanation:
Answer:
number of negative electric charger
explanation
(the electrons ) and positive electric charges (the protons). The total electric charge of the atom is therefore zero and the atom is said to be neutral .
The formulas for ethane, ethene, and ethyne are c2h6 , c2h4 , and c2h2 , respectively. Rank these compounds by the length of the carbonâ€"carbon bond.
Answers
The descending order of carbon-carbon bond length in C₂H₆, C₂H₄, and C₂H₂ is:-
C₂H₆, C₂H₄, C₂H₂
Hydrocarbons are compounds that consist of only hydrogen and carbon.
On the basis of the presence or absence of double bonds/triple bonds, they are of three types- alkanes, alkenes, and alkynes.
Alkanes do not contain any double bonds.Alkenes contain one or more double bond(s) between the carbon atoms.Alkenes contain one or more triple bond(s) between carbon atoms.The comparison between alkanes, alkenes, and alkynes is given in the adjoining image.
We can see that the bond length in alkanes like C₂H₆ is 1.53 A which is greater than alkenes(C₂H₄) , 1.33 A which is still greater than alkynes(C₂H₂) with a value of 1.18 A.
Thus, The descending order of carbon-carbon bond length in C₂H₆, C₂H₄, and C₂H₂ is:
C₂H₆, C₂H₄, C₂H₂
To know more about "hydrocarbons", refer to the link given below:
brainly.com/question/16858042?referrer=searchResults
#SPJ4

What is the composition of an isotope of calcium (atomic number 20)?
21 protons, 20 neutrons, 20 electrons
20 protons, 25 neutrons, 20 electrons
22 protons, 20 neutrons, 19 electrons
19 protons, 20 neutrons, 19 electrons
Answers
Answer:
The second one
Explanation:
Because the isotopes have the same number of protons and different number of neutrons compared with the original element
Plants prevent a) soil erosion by wind or water b) soil erosion due to a forest station c) The loosening of soil and they bind the soil with the help of the roots
Answers
Answer:
The loosening of soil and they bind the soil with the help of the roots
Explanation:
Here, we want to know how plants prevent erosion by wind or water.
The way they do this is by binding the soil with their roots such that it will not be easy for the erosion agent to erode the surface.
How they do this is by making sure that the soil
particles are binded together with their roots which helps to keep the particles of the soil intact and not eroded
Answer:
c
Explanation: