Answers
Answer:
D. Uses sunlight to create glucose (sugar)
Explanation:
The function of the chloroplast is that it uses sunlight to create glucose.
The chloroplast allows for light to be trapped which is used for the synthesis of glucose from carbon dioxide and water.
The chloroplast is a rich in a pigment called the chlorophyll. This chlorophyll is a green pigment which allows for the trapping of solar energy
This solar energy is used to carryout the photosynthetic reaction.
Related Questions
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
Consider 1.56 grams of H2(g) produced by the following chemical reaction. 2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g) Determine if each of the following statements is True or False. The reaction requires 27.9 grams of H2O. [ Select ] The reaction also produces 1.55 grams of NaOH. [ Select ] The grams consumed will equal the grams produced in this chemical reaction.
Answers
The actual amount of substance each required or produced by the reaction is obtained by stoichiometry.
From the reaction equation;
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Number of moles in 1.56 grams of H2(g) = 1.56 grams/2.00g/mol = 0.78 moles
If 2 moles of water yields 1 mole of H2
x moles of water yields 0.78 moles of H2
x = 2 × 0.78/1 = 1.56 moles of water
Mass of water required = 1.56 moles of water × 18 g/mol = 28 grams of water
The statement that the reaction requires 27.9 grams of H2O is true.
Also;
The number of moles in 1.55g of NaOH = 1.55g/40g/mol = 0.039 moles
If 2 moles of water produces 2 moles of NaOH
1.56 moles of water produces 1.56 × 2/2 = 1.56 moles of NaOH
Mass of NaOH = 1.56 moles of NaOH * 40 g/mol = 62.4 g of NaOH
The statement that the reaction also produces 1.55 grams of NaOH is false.
Learn more: https://brainly.com/question/9743981
A solution of copper sulfate is treated with zinc metal. How many grams of copper are produced if 2.9 g of zinc are consumed? (Hint: Make sure to balance the equation first)
Select one:
a.
2.9 g
b.
2.8 g
c.
5.7 g
d.
3.7 g
Answers
Answer:
b. 2.8
Explanation:
To determine the amount of copper produced, we need to first balance the chemical equation for the reaction between copper sulfate and zinc. The balanced equation is as follows:
Zn + CuSO4 → ZnSO4 + Cu
From the balanced equation, we can see that 1 mole of zinc (Zn) reacts with 1 mole of copper sulfate (CuSO4) to produce 1 mole of copper (Cu). The molar mass of zinc is 65.38 g/mol, and the molar mass of copper is 63.55 g/mol.
Given that 2.9 g of zinc is consumed, we can calculate the moles of zinc:
moles of zinc = mass of zinc / molar mass of zinc
= 2.9 g / 65.38 g/mol
≈ 0.0443 mol
Since the reaction is 1:1 between zinc and copper, the moles of copper produced will be the same as the moles of zinc consumed. Therefore, 0.0443 mol of copper is produced.
Now, we can calculate the mass of copper:
mass of copper = moles of copper × molar mass of copper
= 0.0443 mol × 63.55 g/mol
≈ 2.81 g
Therefore, the correct answer is b. 2.8 g
chatgpt
The balanced equation for the reaction is:
Zn + CuSO4 -> ZnSO4 + Cu
This means that 1 mole of zinc reacts with 1 mole of copper sulfate to produce 1 mole of zinc sulfate and 1 mole of copper.
The molar mass of zinc is 65.38 g/mol, and the molar mass of copper is 63.55 g/mol.
Therefore, 2.9 g of zinc is equivalent to 0.044 moles of zinc.
This means that 0.044 moles of copper will be produced.
The molar mass of copper is 63.55 g/mol, so 0.044 moles of copper is equivalent to 2.8 g of copper.
Therefore, 2.8 g of copper will be produced.
So the answer is (b).
bardAI
What mass (grams) of sodium sulfate would be formed by the complete reaction of 120.0 grams of sodium hydroxide?
Answers
Answer:
The mass of sodium sulfate formed by the comolete reaction of 120.0 grams of sodium hydroxide is 142.04 grams.
Explanation:
The balanced chemical equation for the reaction between sodium hydroxide and sulfuric acid is:
2NaOH + H2SO4 -> Na2SO4 + 2H2O
From the equation, we can see that 2 moles of sodium hydroxide react with 1 mole of sulfuric acid to form 1 mole of sodium sulfate. We can use this information, along with the molar masses of the compounds, to calculate the mass of sodium sulfate formed.
First, we need to convert the given mqss of sodium hydroxide to moles. The molar mass of sodium hydroxide is 40.00 g/mol, so:
Moles of NaOH = Mass of NaOH / Molar mass of NaOH
Moles of NaOH = 120.0 g / 40.00 g/mol
Moles of NaOH = 3.00 mol
Next, we can use the mole ratio from the balanced equation to calculate the moles of sodium sulfate formed:
Moles of Na2SO4 = Moles of NaOH / 2
Moles of Na2SO4 = 3.00 mol / 2
Moles of Na2SO4 = 1.50 mol
Finally, we can convert the moles of sodium sulfate to grams using its molqr mass of 142.04 g/mol:
Mass of Na2SO4 = Moles of Na2SO4 x Molar mass of Na2SO4
Mass of Na2SO4 = 1.50 mol x 142.04 g/mol
Mass of Na2SO4 = 213.06 g
Therefore, the mass of sodium sulfate formed by the complete reaction of 120.0 grams of sodium hydroxide is 213.06 grams.
The theory of plate tectonics helps explain the location of volcanoes and earthquakes. Which of these also describes the current theory of plate tectonics?
ANSWERS
° it combines elements of continental drift and seafloor spreading.
° it suggests that the lithosphere is divided into pieces, called plates. °
denser ocean crust sinks below less-dense continental crust along subduction zones.
° all of the above.
Answers
The theory of plate tectonics explains the location of most earthquakes and volcanoes as on the boundaries of tectonic plates, most of the earthquakes or volcanoes happen.
What are plate tectonics?Plate tectonics is the plates that are present in the earth's crust. These plates are of different types. These plates are always in movement. These plates are divergent, convergent, etc.
Earthquakes, volcanoes, and tectonic plates. The plate tectonics hypothesis states that Earth is a dynamic planet.
The numerous separate plates that make up its surface move and interact with one another, continually modifying and reshaping the Earth's outer layer. Tectonic plate movement causes both earthquakes and volcanoes.
Thus, the ends of the tectonic plates are the places where earthquakes or volcanoes happen.
For the following reaction, 22.3 grams of sulfur dioxide are allowed to react with 5.42 grams of water.
sulfur dioxide (g) + water (l)→ sulfurous acid (H₂SO3) (g)
What is the maximum amount of sulfurous acid (H₂SO3) that can be formed? 24.7 grams
What is the FORMULA for the limiting reagent? H₂O
What amount of the excess reagent remains after the reaction is complete? 3.01 grams
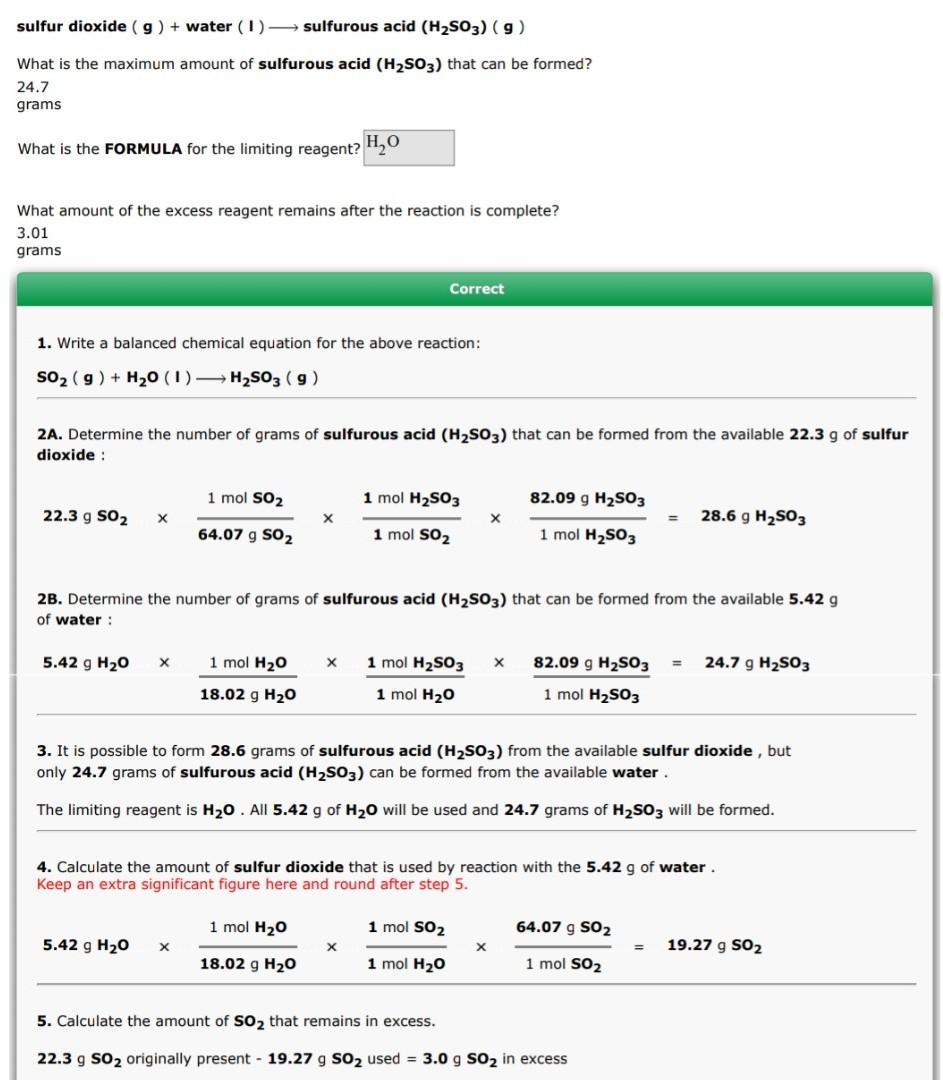
Answers
Explanation:
# grams Sulfuric Acid formed from 22.3 g Sulfur Dioxide
\(23.2 \: g \: SO₂ \times \frac{1 \: mol \: SO₂)}{64.07g \: SO₂} \times \frac{1 \: mol \: H₂SO₃}{1 \: mol \: SO₂)} \times \frac{82.09 \: g \: H₂SO₃}{1 \: mol \:SO₂ } = 28.6 \: g \: H₂SO₃\)
# grams Sulfuric Acid formed from 5.42 g Water
\(5.42 \: g \: SO₂ \frac{1 \: mol \: H₂O)}{18.02g \: H₂O} \times \frac{1 \: mol \: H₂SO₃}{1 \: mol \: H₂O)} \times \frac{82.09 \: g \: H₂SO₃}{1 \: mol \:H₂SO₃ } = 24.7 \: g \: H₂SO₃\)
# Sulfur Dioxide used by reaction 5.42 g Water
\(5.42 \: g \: H₂O \times \frac{1 \: mol \: H₂O)}{18.02g \: H₂O} \times \frac{1 \: mol \: SO₂}{1 \: mol \: H₂O)} \times \frac{64.07 \: g \: SO₂}{1 \: mol \:SO₂ } = 19.27 \: g \: SO₂\)
# of SO₂ in Excess
\( 23.2 \: g \: SO₂ - 19.27 \: g \: SO₂ = 3.0 \: g \: SO₂\)
Which of the following qualities is most frequently used to measure the amount of a substance in a solution?
weight
shape
size
density
light absorbance
Answers
There are many methods which can be used to represent the concentration of a solution. Molarity, mole fraction, molality, etc. are used to represent the concentration of a solution. Here weight denotes the quantity of the substance. The correct option is A.
What is concentration?The concentration of a substance is defined as the quantity of the solute present in a given amount of the solution. The quantity of a solute is usually denoted as the weight of the solute. The method molarity is mainly based on the weight of the solute.
The amount of substance can be represented by weight and its units are gram and kilogram.
Thus the correct option is A.
To know more about weight, visit;
https://brainly.com/question/7152617
#SPJ1
3.Calculate the pH of a 0.250 M solution of potassium phenolate, KC6H5O. Ka for phenol (C6H5OH) is 1.0 x 10-10.
Answers
From the statement and the information available in the question, the pH of the solution is 12.2.
What is Ka?The term Ka refers to the acid dissociation constant of a substance, we can obtain this by setting up the ICE table as shown;
C6H5O^- + H2O ⇄ C6H5OH + OH^-
I 0.250 0 0
C -X +X +X
E 0.250 -x x x
Now;
Kb = 1 * 10^-14/ 1.0 x 10-10
Kb = 1 * 10^-4
Kb = [C6H5OH] [OH^-]/[C6H5O^-]
1 * 10^-4 = x^2/ 0.250 -x
1 * 10^-4 (0.250 -x) = x^2
2.5 * 10^-4 - 1 * 10^-4 x = x^2
x^2 + 1 * 10^-4 x - 2.5 * 10^-4 = 0
x=0.016 M
Given that; [C6H5OH] = [OH^-] =x
pOH = -log(0.016 M) = 1.8
pH = 14 - 1.8 = 12.2
Learn more about Ka: https://brainly.com/question/16236454
how does the name of CaS
differ from the name of CdS?
Answers
Answer:
Ca is calcium
Cd is cadmium
Ca in Grp 2
Cd is transition metal
...
How many moles is 3.01 x 10 24 molecules of oxygen ( g)?
Answers
Answer:
5.00 moles O₂
General Formulas and Concepts:
Chemistry - Atomic Structure
Using Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:
Step 1: Define
3.01 × 10²⁴ molecules O₂
Step 2: Convert
\(3.01 \cdot 10^{24} \ mc \ O_2(\frac{1 \ mol \ O_2}{6.022 \cdot 10^{23} \ mc \ O_2} )\) = 4.99834 moles O₂
Step 3: Check
We are given 3 sig figs. Follow sig fig rules and round.
4.99834 moles O₂ ≈ 5.00 moles O₂
calculate the kilojoules needed to heat 175g of copper from 26°c to 184°c
Answers
Answer:
The answer is 10.507 KJ
Explanation:
The formula you need to use for this question is MC(delta)T:
M=Mass ( 175g)
C=Specific Heat ( depends on the element, copper=0.38 j/g.k )
and T= Temperature, where you find the change in temperature by minusing the final T with the initial T.
so, (175g)(0.38)(184-26)= 10,507 J
since we need the answer in KJ divide 10,507 by 1000
10,507/1000 = 10.507 KJ
Identify the type of reaction. Complete the equations with the correct reactants then balance each equation.

Answers
The balanced equations are Cd (g) + S\(_8\) (g) → CdS (g), K\(_2\)CO\(_3\) → K\(_2\)O + CO\(_2\). and a Na + HOH → NaOH + H\(_2\). Cd (g) + S\(_8\) (g) → CdS (g) is combination reaction.
An equation per a chemical reaction is said to be balanced if both the reactants plus the products have the same number of atoms and total charge for each component of the reaction. In other words, both sides between the reaction have an equal balance of mass and charge. The balanced equations are Cd (g) + S\(_8\) (g) → CdS (g), K\(_2\)CO\(_3\) → K\(_2\)O + CO\(_2\). and a Na + HOH → NaOH + H\(_2\).
Cd (g) + S\(_8\) (g) → CdS (g) = combination reaction
K\(_2\)CO\(_3\) → K\(_2\)O + CO\(_2\) = decomposition reaction
Na + HOH → NaOH + H\(_2\) = Hydration reaction
To know more about balanced equation, here:
https://brainly.com/question/7181548
#SPJ1
1H
35Cl has a force constant (k) value of 480 Nm-1
. Calculate the fundamental frequency
and its wavenumber.
Answers
The wave number of the compound can be calculated as shown and we are going to have 8.651 x 10^13 s^-1.
What is the force constant of molecule?The force constant of a molecule is a measure of the strength of the chemical bonds between the atoms in the molecule. It is defined as the second derivative of the potential energy of the molecule with respect to atomic displacements, evaluated at the equilibrium geometry of the molecule.
μH35Cl = 1.627 x 10^-27 kg
v = 1/2π x (480.5kg m s^-2 / 1.627 x 10^-27 kg)^1/2
v = 0.1592 x (2.953 x 10^29 m s^-2)^1/2
v = 0.1592 x (5.434 x 10^14 s^-1)
v = 8.651 x 10^13 s^-1
Learn more about force constant:https://brainly.com/question/30951247
#SPJ1
A mixture of 0.224 g of H2, 1.06 g of N2, and 0.834 g of Ar is stored in a closed container at STP. Find the volume (in L) of the container, assuming that the gases exhibit ideal behavior.
Answers
Answer: The volume of given container is 3.83 L.
Explanation:
Given: Mass of \(H_{2}\) = 0.224 g
Mass of \(N_{2}\) = 1.06 g
Mass of Ar = 0.834 g
Since, moles is the mass of a substance divided by its molar mass. Therefore, moles of given substances present in the mixture are as follows.
Moles of \(H_{2}\) are:
\(Moles = \frac{mass}{molar mass}\\= \frac{0.224 g}{2 g/mol}\\= 0.112 mol\)
Moles of \(N_{2}\) are:
\(Moles = \frac{mass}{molar mass}\\= \frac{1.06 g}{28 g/mol}\\= 0.038 mol\)
Moles of Ar are:
\(Moles = \frac{mass}{molar mass}\\= \frac{0.834 g}{40 g/mol}\\= 0.021 mol\)
Total moles = (0.112 + 0.038 + 0.021) mol = 0.171 mol
Now, using ideal gas equation the volume is calculated as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(V = \frac{nRT}{P}\\= \frac{0.171 mol \times 0.0821 L atm/mol K \times 273 K}{1 atm}\\= 3.83 L\)
Thus, we can conclude that the volume of given container is 3.83 L.
What is the energy of an electron in a Li+ ion when an electron moves from n = 2 to n =3?
Answers
Answer:
The question wants you to determine the energy that the incoming photon must have in order to allow the electron that absorbs it to jump from
n
i
=
2
to
n
f
=
6
.
A good starting point here will be to calculate the energy of the photon emitted when the electron falls from
n
i
=
6
to
n
f
=
2
by using the Rydberg equation.
1
λ
=
R
⋅
(
1
n
2
f
−
1
n
2
i
)
Here
λ
si the wavelength of the emittted photon
R
is the Rydberg constant, equal to
1.097
⋅
10
7
m
−
1
Plug in your values to find
1
λ
=
1.097
⋅
10
7
.
m
−
1
⋅
(
1
2
2
−
1
6
2
)
1
λ
=
2.4378
⋅
10
6
.
m
−
1
This means that you have
λ
=
4.10
⋅
10
−
7
.
m
So, you know that when an electron falls from
n
i
=
6
to
n
f
=
2
, a photon of wavelength
410 nm
is emitted. This implies that in order for the electron to jump from
n
i
=
2
to
n
f
=
6
, it must absorb a photon of the same wavelength.
Solution Notes
8. Calculate the molarity of 500 ml of 0.0300 moles of NaOH.
Answers
Answer:
\(\huge\boxed{\sf M = 0.06\ M}\)
Explanation:
Given data:No. of moles = n = 0.03 mol
Volume = v = 500 ml = 0.5 L
Required:Molarity = M = ?
Formula:M = n / v
Solution:Put the given data in the above formula.
M = 0.03 / 0.5
M = 0.06 M\(\rule[225]{225}{2}\)
The single strand of nucleic acid shown is representative of
A). RNA
B). DNA
C). both RNA and DNA
D). protein

Answers
witch color of light has the least energy?
Answers
Answer: Red
Explanation:
Question in picture
Question in picture

Answers
The correct answer is a sphybridisation in z coordinate.So to form sphybridisation we need a s orbital and a p orbital .
In genomics, hybridization is the process by which two complementary single-stranded DNA or RNA molecules bond together to form a double-stranded molecule.The bonding is determined by the correct base pairing between the two single-stranded molecules. When one s and one p orbital in the same main shell of an atom combine to form two new equivalent orbitals, this is referred to as sphybridization.
The newly formed orbitals are known as sphybridized orbitals. It forms linear molecules with a 180° angle. Atomic orbitals include both s and p orbitals. These orbitals represent the most likely region in which we can find an electron of that atom. The primary distinction between s and p orbitals is that s orbitals are spherical in shape, whereas p orbitals are dumvell-shaped.So to form sp hybridisation we need a s orbital and a p orbital .
Learn more about hybridisation here :-
https://brainly.com/question/14140731
#SPJ9

Which element requires the LEAST amount of energy to remove the most loosely held electron from a gaseous atom in the ground state?
Which element requires the LEAST amount of energy to remove the most loosely held electron from a gaseous atom in the ground state?
K,Br,Ti, Copper
Answers
Answer:
K / Potassium
Explanation:
Out of the list of elements provided, the element that requires the least amount of energy to remove the most loosely held electron from a gaseous atom in the ground state is K (potassium).
The amount of energy needed to remove an electron from an atom is known as the ionization energy. The ionization energy of an element depends on the atomic structure of the element, specifically the arrangement of electrons in the atom's outermost energy level (valence shell). Elements with a low ionization energy have valence electrons that are held less tightly to the nucleus, making them easier to remove.
In general, the ionization energy of an element increases as you move from left to right across a period (row) of the periodic table. This trend is due to the increasing nuclear charge (number of protons in the nucleus) as you move across the period, which leads to a stronger attraction between the nucleus and the valence electrons. However, there are some exceptions to this trend, such as K (potassium), which has a lower ionization energy than its neighbors on the periodic table (Ca and Sr).
Therefore, out of the elements listed (K, Br, Ti, and Cu), K has the lowest ionization energy and would require the least amount of energy to remove the most loosely held electron from a gaseous atom in the ground state.
How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity can create a magnetic field.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.
Need help now this work is due by 5:00
Answers
Answer: Electricity and magnetism are essentially two aspects of the same thing, because a changing electric field creates a magnetic field, and a changing magnetic field creates an electric field.
Answer:How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.How are electricity and magnetism related?
Moving magnets can make electrical currents.
Electricity and magnetism are not related.
Protons and electrons are repelled by magnets.
Explanation:
More than two thirds of elements are classified as
Answers
Answer:
Metals
Explanation:
Two Thirds means 75 %.
Metals account for about more than two - thirds of all elements.
Cumulative Exam Active
41 42 43 144
The electron configuration of nitrogen (N) is
O 1s²2s²2p³
O 1s²2s²2p4
O 1s²2s²2p5
O 1s²2s²2p6
Answers
The answer is: The electronic configuration of Nitrogen is \(1s^22s^22p^3\).
Electronic configuration: The electronic configuration is defined as the distribution of electrons of an atom in the atomic or molecular orbitals and is written using the labels for the subshell.
How to decide which orbital is filled first?
The order in which electrons are filled in atomic orbitals as:(Shown in image)
Just follow the arrows to select the orbitals, s orbital can have 2 electrons, p can have 6 electrons, d can have 10 electrons and f can 14 electrons.The electronic configuration in which the outer shell is completely filled is known as noble-gas configuration as they are similar to electronic configurations of noble gases.Now, the given element is nitrogen (\(N\)). The atomic number of Nitrogen is 7. Thus, these 7 electrons are filled as-\(1s^22s^22p^3\)
Therefore, the electronic configuration of Nitrogen is \(1s^22s^22p^3\).To learn more about the electronic configuration, visit:
https://brainly.com/question/21977349
#SPJ4

Nitrogen's complete electron configuration is 12s2s22p3.
The shorthand electron configuration for noble gases is [He] 2s22p3. Nitrogen has an atomic number of 7. The nitrogen atoms' nucleus contain this many protons. An atom that is neutral has an equal number of protons and electrons. Thus, the ground state electron configuration will consist of 7 electrons in the suitable s and p orbitals (state of lowest energy). For nitrogen, the entire electron configuration is 1s22s22p. Scientists may easily express and explain how the electrons are organized around the nitrogen atom's nucleus by using the configuration notation for nitrogen (N). As a result, it is simpler to comprehend and forecast how atoms will cooperate to form chemical bonds.
Learn more about electronic configuration here-
https://brainly.com/question/11309892
#SPJ9
⁷ 168 g of iron reacts with excess oxygen to form 232 g of an oxide of iron. iron + oxygen H ratio iron oxide a. Calculate the mass of oxygen in the iron oxide. b. Calculate the males of oxygen in the oxide. c. Calculate the moles of iron in the iron oxide. d. Calculate the ratio of the iron to oxygen to give the formula of this oxide of iron. formula
Answers
The mass of the oxygen that reacted is 70.4 g.
What is the reaction of iron and oxygen?
The reaction between iron and oxygen is called rusting and is a oxidation process. The balanced chemical equation for the reaction is:
4 Fe + 3 O2 -> 2 Fe2O3
Number of moles of iron = 168 g/56 g/mol = 3 moles
Number of moles of iron III oxide = 232 g /160 g/mol
= 1.45 moles
The mass of the oxygen that reacted is;
If 3 moles of oxygen produces 2 moles of oxide
x moles of oxygen would produce 1.45 moles ofn the oxde
x = 3 * 1.45/2
= 2.2 moles
Mass of the oxygen = 2.2 moles * 32 g/mol
= 70.4 g
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1
How many grams of hydrogen gas will be produced if 2.5 moles of tin react?
Answers
Answer:
I believe it's 5.0 g of hydrogen
Explanation:
Sorry if i'm wrong
when coal goes through combustion it releases nitrogen and sulpur into the air in the form of what
Answers
Answer:
Over time, the plant matter transforms from moist, low-carbon peat, to coal, All types of coal also contain sulfur, which, when burned, releases toxic air pollution. Sulfur All this coal comes from mines, which are either underground or run on diesel—a major source of air toxins, nitrogen oxide, and soot.
Find the grams in 6.25 moles of HC2H302.
Answers
use this formula m=n×M
Your n would be 6.25mols
Your Molar mass would be the number you get after adding the molar mass of your equation: HC2H302. Find your periodic table and look there.
And you plug in the molar number in the formula and you multiply n×M
I would help but I don't have the periodic table with me right now
What is the energy of a photon of wavelength 5.50 x 10(-7) m in Joules?
Answers
The answer is 1.09 x \(10^{-35}\) J
To find the energy of a photon with a given wavelength,
Substitute the wavelength 5.50 x 10(-7)Planck's constant in joules (h = 6.6261 1034 J/s), The speed of light (c = 299792458 m/s) into equationE = h x c x λ
You'll obtain an energy result in joules using these units (J).
According to the question,
wavelength, λ = 5.50 x \(10^{-7}\)
speed of light, c = 3 x \(10^{8}\) m/s
Planck's constant, h = 6.62607015 x \(10^{-34}\)
E = 6.62607015 x \(10^{-34}\) x 3 x \(10^{8}\) x 5.50 x \(10^{-7}\)
E = 1.09 x \(10^{-35}\) J
Therefore, the energy of a photon is 1.09 x \(10^{-35}\) J
To know more about the energy of photons:
brainly.com/question/16387660
When converting from moles into atoms, what number should you put on the top of the conversation factor?

Answers
The number of the atoms that should be the numerator is 6.02 * 10^23 atoms.
What is the conversion factor?We have to note that the conversion factor is the factor that can be used in the conversion of one unit to the other. We have to note that if we are able to change the unit then we can be able to make the conversion.
We have to note that the Avogadro's number is the number of atoms that we can find in one mole of a substance and it has a value of about 6.02 * 10^23 atoms in the atom of magnesium.
Learn more about atoms:https://brainly.com/question/13654549
#SPJ1
What is the boiling point in °C of a 0.32 molal aqueous solution of NaCl?
BP (water) = 100.00 °C Kb (Water) = 0.512 °C/m
Answers
Answer:
the boiling point of solution at 3 decimal point is 100.329०C Ans.
Explanation:
given data -
molality of Nacl = 0.321 m
molal boiling point elevation constant (Kb) =0.512०C/m
# formula of change of boiling point of sample =
∆ Tb =i × Kb × m
Kb = molal boiling point of elevation constant
m = molality
i = vont's hoff factor.
Nacl is strong electrolyte and its 100% dissociate so the value of i for Nacl is 2
put value in the formula
∆ Tb = 2 × 0.512 ०C/m × 0.321m
= 0.3287
= 0.329०C
∆Tb = T'b - Tb
T'b = boiling point of solution
Tb= boiling point of solvent( water)
0.329०C = T'b - 100०c ( boiling point of water = 100०C)
T'b = 0.329०C + 100०C
= 100.329०C
hope this helps