What is the difference between an open chemical system system and a closed chemical system?.
Answers
Chemical systems are commonly divided into two categories, open systems and closed systems. The primary distinction between open and closed systems is their capacity to transmit matter or energy. In this section, we'll look at the key distinctions between open and closed chemical systems.
When chemical reactions occur, they usually take place in an environment that contains other substances. The extent to which these substances can affect the outcome of the reaction is determined by the degree of interaction between the system and its surroundings.
Closed chemical systems are referred to as systems that don't permit the transfer of matter, although they can exchange energy. In contrast, open chemical systems are those that allow both matter and energy to be exchanged with their environment.
To know more about systems visit:
https://brainly.com/question/19843453
#SPJ11
Related Questions
Determine the product(s) formed when cyclohexene is treated with the following reagents
Answers
Explanation:
Big C carbon surrounded by He helium
How many atoms are in 3CaCO3
Answers
Answer:
15
Explanation:
3 Calcium
3 Carbon
9 Oxygen
sample with masses 0.12g,1.8g, and 0.562g are mixed together the combind mass of the three samples expressed to the correct number of significant figures should be recorded as
Answers
Answer:
2.5 g
Explanation:
Given data:
Masses of sample = 0.12 g, 1.8 g, 0.562 g
Combine mass of samples = ?
Solution:
When we add or subtract the values the number of significant figures after decimal in result must be equal to the given measurement having less number of decimal places.
0.12 g + 1.8 g + 0.562 g
2.482 g
In given three measurements 1.8 has less number of significant figure after decimal point which is only one digit. Thus the final value must contain one digit after decimal.
we will round of 2.482 g.
2.5 g
because the next digit after 4 is 8 that's why we will round 4 to 5.
What chemical process is responsible for the smell of vinegar in an old bottle of aspirin?
Answers
Chemical process is responsible for the smell of vinegar in an old bottle of aspirin is Hydrolysis of ester i.e. Aspirin.
Aspirin reacts with water leading to the formation of Acetic acid which is a Carboxylic acid derivative.
Aspirin on Hydrolysis forms Acetic acid and Salicylic acid.
The reaction shows up as a fizz when aspirin is added in water.
Aspirin is called Acetyl Salicylic acid and it helps to reduce:
InflammationPainFeverHeadacheArthritisMuscle painTooth painAcetic acid or Ethanoic acid is a colorless liquid. It is an important carboxylic acid. It smells like vinegar.
Acetic acid is used for production of following:
Vinyl AcetateInsecticidesRubberAcetic anhydrideSalicylic acid is a bitter compound which is colorless. It reduces acne and opens up the skin pores.
Learn more about Aspirin here, https://brainly.com/question/23878261
#SPJ4
Influx of ____ or _____ ions result in EPSPs.
A) Ca+; K+
B) Na+; Ca2+
C) Cl-; Na+
D) Ca2+; Cl-
Answers
The correct answer is:
B) Na⁺; Ca²⁺ for influx of ions.
An influx action potential that enters a presynaptic terminal activates Ca2+ channels and momentarily raises the local Ca2+ concentration in the presynaptic active zone. After activating synaptotagmins Ca2+, neurotransmitter release occurs within a few hundred microseconds. Through the interaction of their C2-domains with phospholipids and SNARE proteins, synaptotagmins' two C2-domains bind Ca2+ and translate the Ca2+ signal into a nanomechanical activation of the membrane fusion machinery. Synaptotagmins cannot initiate exocytosis on their own; instead, they need a necessary cofactor known as complexin, a tiny protein that binds to SNARE complexes and simultaneously activates and clamps the SNARE complexes, setting them up for later synaptotagmin action.
To know more about influx:
https://brainly.com/question/12858264
#SPJ4
help me please!
Thermal energy can come from other forms of energy. Give several examples how.
i'm getting graded for this!!!
Answers
If it requires 75.0mL of 0.500M NaOH to neutralize 165.0 mL of an hcl solution what is the concentration of the hcl solution
Answers
Answer:
0.027 M HCl
Explanation:
The chemical equation of the neutralization is:
1 NaOH + 1 HCl -> 1 H2O + 1 NaCl
Because the ratio of NaOH and HCl is 1:1 you can use the M1V1=M2V2 formula.
(75 mL)(0.5 M NaOH) = (165 mL)(M HCl)
It requires 0.027 M HCl.
How many grams are present in 2.5 moles of glucose (C6H12O6)?
Answers
Hmmmmm
Hmmmmmm
Hmmmmmmm
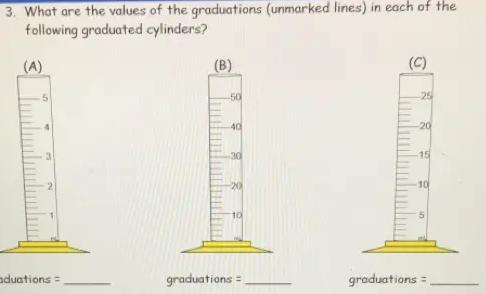
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
a scientist wants to make a solution of tribasic sodium phosphate, na3po4 , for a laboratory experiment. how many grams of na3po4 will be needed to produce 325 ml of a solution that has a concentration of na ions of 1.40 m ?
Answers
The researcher will require 187.66 grammes of Na3PO4 to create a 325 ml solution with a Na ion concentration of 1.40 M.
We must take the molar concentration and volume of the desired solution into account in order to calculate the required amount of Na3PO4.
1.40 M, or 1.40 moles of Na ions per litre of solution, is the value for the molar concentration, or M. Since 325 ml (0.325 L) is required, we may determine how many moles of Na ions are required:
Molar concentration times volume equals the number of moles. For example, 1.40 mol/L times 0.325 L equals 0.455 mol
The chemical structure of tribasic sodium phosphate (Na3PO4) must therefore be taken into account. According to the formula, there are 3 moles of Na ions for every mole of Na3PO4.
As a result, one-third as many moles of Na3PO4 are required as there are of Na ions:
The formula for Na3PO4's moles is 0.455 mol / 3 = 0.1517 mol.
Finally, we may use the molar mass of Na3PO4 to determine the required mass. Na3PO4 has a molar mass of approximately 163.94 g/mol:
Mass equals moles times molar mass.
Mass = 163.94 g/mol x 0.1517 mol, or 24.86 g
As a result, the researcher will require about 24.86 grammes of Na3PO4 to make a 325 ml solution with a 1.40 M concentration of Na ions.
To know more about "Concentration" refer here:
https://brainly.com/question/30639206#
#SPJ11
Consider two 5 l chambers. In one, there are 5. 00 g o₂, and in the other there are 5. 00 g he. Which has the higher pressure at room temperature?.
Answers
The gas having higher pressure at room temperature, is P = 6.11 atm.
What is the ideal gas law?
The general gas equation, often known as the ideal gas law, is the equation of state for a hypothetical ideal gas. It has a number of limitations, but it provides a decent approximation of the behavior of numerous gases under various circumstances.
According to the ideal gas equation,
PV =nRT
For O₂,
Number of moles of oxygen gas = 5.00 g /32 g/mol = 0.156 moles
P =?
V = 5 L
n = 0.156 moles
T = 25 + 273 = 298 K
R = 0.082 atmLK-1mol-1
P = nRT/V
P = 0.156 moles × 0.082 atmLK-1mol-1 × 298 K/5 L
P = 0.76 atm
For He,
Number of moles of He = 5/4 g/mol = 1.25 moles
P =?
V = 5 L
n = 1.25 moles
T = 25 + 273 = 298 K
R = 0.082 atmLK-1mol-1
P = nRT/V
P =1.25 moles × 0.082 atmLK-1mol-1 × 298 K/5 L
P = 6.11 atm
Hence the gas having higher pressure at room temperature is He.
To learn more about gas laws follow the link:
https://brainly.com/question/25290815
#SPJ4
The rays that damage the skin are call?
Answers
Answer:
ultraviolet, or UV rays from the sun can damage yor skin. thats why you put on sunscreen lol
2. You have 200g or a solution that contains 30g of hydrochloric acid (HCI),
what percentage of your solution is made up of HCI acid?
Answers
Answer:
the percentage of your solution that made up of HCI acid is 15%
Explanation:
The computation of the percentage of your solution that made up of HCI acid is given below:
Given that
There is 200g or a solution that have 0 g of hydrochloric acid (HCI)
Based on the above information
The percentage is
= 30g ÷ 200g
= 15%
Hence, the percentage of your solution that made up of HCI acid is 15%
What makes an atom radioactive?
Answers
Answer:
The unstable nucleus of radioactive atoms emit radiation.
Explanation:
When the atoms of an element have extra neutrons or protons it creates extra energy in the nucleus and causes the atom to become unbalanced and unstable, or radioactive.
(https://www.epa.gov/radtown/radtown-radioactive-atom-activity-4-atomic-stability)
Answer:
When the atoms of an element have extra neutrons or protons it creates extra energy in the nucleus and causes the atom to become unbalanced or unstable. Whether radioactive elements can become stable and if so, how. The unstable nucleus of radioactive atoms emit radiation. ... This process is called radioactive decay.
Explanation:
In the bromination reaction of your chalcone, you form a racemic mixture. a. Draw the structure of the enantiomers you form and assign R, and S to each of the stereocenters. b. Will you see 2 spots on a TLC of your reaction products
Answers
(a). R-enantiomer: (S)-2-bromo-1-(4-methoxyphenyl)propan-1-one
S-enantiomer: (R)-2-bromo-1-(4-methoxyphenyl)propan-1-one
(b). Yes, you will see two spots on a TLC of your reaction products, one
for each enantiomer
(A) The bromination reaction of chalcone results in the formation of two enantiomers, which are mirror images of each other. Therefore, the R and S configuration will be assigned to each stereocenter. The structure of the enantiomers can be drawn as follows:
R-enantiomer: (S)-2-bromo-1-(4-methoxyphenyl)propan-1-one
S-enantiomer: (R)-2-bromo-1-(4-methoxyphenyl)propan-1-one
(B) Yes, you will see two spots on a TLC of your reaction products, one for each enantiomer. As the enantiomers have different physical and chemical properties, such as boiling points, polarity, and solubility, they will travel at different rates on the TLC plate and thus appear as separate spots.
To know more about enantiomer ,click here:
https://brainly.com/question/6249935
#SPJ11
Synthesis of Aspirin
Discussion – Q&A:
Explain why sodium bicarbonate is added during the work up
Write a complete reaction mech. For prep of aspirin
Explain why crystals during 1st filtration are washed w cold water
Discuss percent yield of reaction
Comment on mp of newly synthesized aspirin
Answers
1. Sodium bicarbonate is added during the work-up phase because it helps in converting any residual acetic anhydride into acetic acid and neutralizes the unreacted salicylic acid.
Sodium bicarbonate is an effective pH neutralizer. In the preparation of aspirin, after the completion of the reaction, hydrochloric acid is added to lower the pH of the reaction mixture to about 2. At this point, aspirin precipitates as it is relatively insoluble in water. After filtration, the crude product is dissolved in hot water. At this stage, sodium bicarbonate is added to neutralize the acidic impurities like the acetic acid that is produced in the reaction. The impurities become soluble and easily removed from the solution.
2. The complete reaction mechanism for the preparation of aspirin is:
3. The crystals are washed with cold water during the first filtration to remove any impurities that may be present. Coldwater is used to prevent the solubility of aspirin in water. This makes it easier to remove any water-soluble impurities and unreacted salicylic acid that may be present.
4. The percent yield of the reaction is calculated by dividing the actual yield obtained by the theoretical yield that is calculated from the stoichiometry of the reactants involved in the reaction. Factors such as incomplete reactions, losses during filtration, and errors in measurement can all contribute to a lower yield. Therefore, the yield may be less than 100%.
5. The melting point of the newly synthesized aspirin should be around 136-140 °C if the reaction was successful. A lower melting point may be an indication of impurities in the final product. The impurities could be from an incomplete reaction, the presence of water or unreacted salicylic acid.
learn more about aspirin here
https://brainly.com/question/25794846
#SPJ11
As molecules increase in electrons, what happens to their melting and boiling points?
Answers
What type of circuit does this figure represent?
A an open parallel circuit
B closed parallel circuit
C open series circuit
D closed series circuit
Answers
Answer:
If it has two resistance one upon another its a parallel circuit but if the resistance move on a line (doesn't matter if it bend ) and if a flap is send open then its a open circuit if the system line connecting the circuit ect is smooth and without any uponing lines them it is a close circuit
Explanation:
Can someone help me

Answers
Answer:
the third option
Explanation:
after you choose that answer don't check if it's correct
◑﹏◐
what is the percent composition of hydrogen in beryllium hydride(BeH2) if 69.6g of beryllium (Be) react with 15.6 g of hydrogen to produce 85.2 g of BeH2
Answers
The percent composition of hydrogen is 18.3%.
What is percent composition?The term percent composition refers to the percentage of a particular component in a compound. It is contained as the ratio of the mass of that component to the total mass multiplied by 100.
From the law of conservation of mass, total mass of beryllium hydride(BeH2) = 85.2 g
Mass of hydrogen = 15.6 g
Percent composition of hydrogen = 15.6 g/ 85.2 g × 100/1 = 18.3%
Learn more about percent composition: https://brainly.com/question/12247957
Give the complete reaction scheme for the catabolism
of Oleoyl-CoA
Answers
The enzyme β-ketothiolase cleaves off the acetyl-CoA molecule from the 3-ketoacyl-CoA, releasing acetyl-CoA and the remaining fatty acid chain forms acyl-CoA, which is two carbons shorter than the original fatty acid chain.
The complete reaction scheme for the catabolism of Oleoyl-CoA is given below:Oleoyl-CoA is broken down into acetyl-CoA, releasing 150 ATP molecules by the process of Beta-oxidation. The complete reaction scheme for the catabolism of Oleoyl-CoA is given below:
Step 1: Oleoyl-CoA is transported to the mitochondria matrix from the cytoplasm with the help of the carnitine shuttle system.
Step 2: The enzyme Acyl-CoA dehydrogenase catalyzes the removal of two hydrogen atoms from the alpha and beta carbons in the fatty acid chain and oxidizes it. This process forms a double bond between the alpha and beta carbon atoms, leading to the formation of trans-Δ2-enoyl-CoA.
Step 3: The enzyme enoyl-CoA hydratase adds a water molecule to the trans-Δ2-enoyl-CoA, converting it into L-3-hydroxyacyl-CoA.
Step 4: The enzyme L-3-hydroxyacyl-CoA dehydrogenase oxidizes L-3-hydroxyacyl-CoA, releasing a hydrogen ion (H+) and two electrons (2e-) and converts it into 3-ketoacyl-CoA.
Step 5: The enzyme β-ketothiolase cleaves off the acetyl-CoA molecule from the 3-ketoacyl-CoA, releasing acetyl-CoA and the remaining fatty acid chain forms acyl-CoA, which is two carbons shorter than the original fatty acid chain.
The cycle starts again, and this process is repeated until the fatty acid chain is completely degraded.
Learn more about fatty acid with the given link,
https://brainly.com/question/17352723
#SPJ11
PLS HELP URGENT
Electron dot diagrams
Use your periodic table to write the electron dot diagrams for the following atoms.
1. Calcium (Ca)
2. Polonium (Po)
3. Moscovium (Mc)
4. Boron (B)
5. Fluorine (F)

Answers

how many grams of tin (ll) fluoride are produced if 45.0 grams HF are reacted
Answers
Approximately 176.3 grams of tin (II) fluoride (SnF2) are produced when 45.0 grams of HF react.
The balanced chemical equation for the reaction between hydrofluoric acid (HF) and tin (II) fluoride (SnF2) is:
2 HF + SnF2 → SnF4 + 2 HCl
This equation tells us that for every 2 moles of HF that react with SnF2, we will get 1 mole of SnF4 produced.
To determine how many grams of SnF2 are produced from 45.0 grams of HF, we need to first convert the mass of HF to moles using its molar mass. The molar mass of HF is approximately 20.01 g/mol:
45.0 g HF × (1 mol HF / 20.01 g HF) = 2.25 mol HF
According to the balanced equation, 2 moles of HF react with 1 mole of SnF2. Therefore, we can determine the moles of SnF2 produced by dividing the moles of HF by 2:
2.25 mol HF ÷ 2 = 1.125 mol SnF2
Finally, we can convert the moles of SnF2 to grams using its molar mass, which is approximately 156.70 g/mol:
1.125 mol SnF2 × (156.70 g SnF2 / 1 mol SnF2) ≈ 176.3 g SnF2
For more question on tin (II) fluoride click on
https://brainly.com/question/29715194
#SPJ11
What would happen if you added Benedict's reagent to solution with sucrose?
Answers
Benedict's reagent to a solution with sucrose, the following will happen:
1. Benedict's reagent, which is a solution of copper sulfate, sodium citrate, and sodium carbonate, will be mixed with the sucrose solution.
2. Since sucrose is a non-reducing sugar, it does not have free aldehyde or ketone groups, which are necessary for the reaction with Benedict's reagent.
3. Therefore, no reaction will occur, and the solution will not change color.
In summary, if you add Benedict's reagent to a solution with sucrose, there will be no observable reaction, as sucrose is a non-reducing sugar.
Benedict's reagent : Alkaline solution of copper sulfate , sodium citrate and sodium carbonate
Glucose and galactose both are reducing sugar. Both of them reduces copper(II) ion in Benedict's reagent into copper (I) ion.
To Learn more about Benedict's reagent:
https://brainly.com/question/30447277
#SPJ11
a stress of 4.75 mpa is applied in the [001] direction of a unit cell of an fcc copper single crystal. determine all the slip system that has highest resolved shear stress.
Answers
The slip systems with the highest resolved shear stress for a stress of 4.75 MPa applied in the [001] direction of an FCC copper single crystal are {110}<111> and {112}<111>.
Which slip systems exhibit the highest resolved shear stress for a stress applied in the [001] direction of an FCC copper single crystal?Slip systems are specific crystallographic planes and directions along which dislocations can occur in a crystal lattice. The resolved shear stress is the component of an applied stress that acts on a particular slip system.
In FCC (face-centered cubic) metals like copper, there are multiple slip systems available for plastic deformation. The {110}<111> and {112}<111> slip systems have been found to exhibit the highest resolved shear stress.
When a stress of 4.75 MPa is applied in the [001] direction of a unit cell of an FCC copper single crystal.
Learn more about Slip systems
brainly.com/question/31665912
#SPJ11
The
force acting on an object is the combination of
all of the individual forces acting on it.
Check it
2 SCRATCHPAD
~ Improve this questio
Answers
Answer:
Yes as you can improve The invisible to human eye force or physical force if any being applied to a object of any mass and with all individual forces as they are acting on this object
Explanation:
Can you define speed for me ?
Answers
Answer:
a rate at which something moves fast
Please help ………………….

Answers
Answer:
magnsium po
Explanation:
What are the parts of an electron configuration?
Answers
Answer:
Explanation:
electronic confirmation=1s^2,2S^2,2P^6,3S^2
atomic no.is 12 therefore :- this is magnesium
Period=3
Block=s-block
Group=II A
A pieces of metal of volume 25cm3 has a Mass of 45g. Determine It density in kg/m3
Answers
The density of the metal is 1800 kg/m^3.
To find the density of the metal in kg/m^3, we need to convert the volume and mass to the appropriate units.
The volume of the metal is given in cm^3, but we need to convert it to m^3:
25 cm^3 = 0.000025 m^3
The mass of the metal is given in grams, but we need to convert it to kilograms:
45 g = 0.045 kg
Now we can use the formula for density, which is:
density = mass / volume
Substituting the values we have:
density = 0.045 kg / 0.000025 m^3
density = 1800 kg/m^3
Therefore, the density of the metal is 1800 kg/m^3.
For more such questions on density visit:
https://brainly.com/question/26364788
#SPJ11