what is the approximate strain energy per ch2 for cyclopropane?
Answers
The approximate strain energy per CH2 for cyclopropane is around 26 kcal/mol. This is due to the high ring strain caused by the geometry of the three-membered ring in cyclopropane, which results in an increased energy requirement to break the carbon-carbon bonds and deform the molecule.
The approximate strain energy per CH2 for cyclopropane is around 27 kcal/mol (113 kJ/mol). Strain energy refers to the additional energy stored in a molecule due to deviations from its ideal geometry, resulting in increased instability. In cyclopropane, the carbon atoms form a planar, equilateral triangle, which leads to bond angles of 60 degrees. This is significantly smaller than the ideal bond angle of 109.5 degrees in sp3 hybridized carbons, causing the high strain energy.
To know more about strain energy Visit:
https://brainly.com/question/28684254
#SPJ11
Related Questions
In an experiment, 5.00 L of N₂ is saturated with water vapor at 22°C and then compressed to half its volume at constant T.
(b) What mass of water vapor condenses to liquid?
Answers
Gas is compressed at a steady temperature without changing the vapour pressure because vapour pressure is a function of temperature.
What exactly is vapour pressure, and why does it exist?The force created as liquids evaporate is known as vapour pressure. Surface area, intermolecular forces, and temperature are three elements that frequently have an impact on vapour press. At various temperatures, molecules have varying vapour pressures.
What factors affect vapour pressure?Temperature is the only factor that affects vapour pressures. The vapour pressure of a liquid is independent of the volume of the liquid in the container, whether it is one litre or thirty litres; both samples will have the same vapour pressure at the same temperature.
Learn more about vapour pressure here:-
https://brainly.com/question/27283213
#SPJ4
what are all of the mole ratios in acetic acid
Answers
The mole ratio between sodium bicarbonate and that of acetic acid is 1:1
What is mole ratio?A mole ratio can be defined as that ratio in whole numbers between the amounts in moles of any two compounds involved in a balanced chemical reaction.
The mole ratio of each individual element is found by dividing the number of moles of each by the smallest number of moles.In conclusion, the mole ratio between sodium bicarbonate and that of acetic acid is 1:1
Learn more about mole ratio:
https://brainly.com/question/19099163
#SPJ1
What are substances that are generally sour and have a pH level of 6.9 and below.
Answers
Answer: Like, milk?
Answer:
the substance are acids.
Explanation:
between 6.0 -6.9 are slightly acidic
Which of the following is true of a catalyst? Check all that apply.
1) it is used up during a reaction
2) in the body, it is often an enzyme
3) it changes the rate of a reaction
4) it affects the rate-limiting step
5) it always changes colors
Answers
A catalyst is a substance that changes the rate of a reaction without being consumed by it. Therefore,
option 1 is not true of a catalyst.
Option 2 is partially correct as enzymes are biological catalysts found in the body.
Option 3 is true, as a catalyst alters the rate of a reaction by lowering the activation energy needed for the reaction to occur.
Option 4 is also true, as a catalyst can affect the rate-limiting step, which is the slowest step in a reaction that determines the overall rate.
Option 5 is not true, as a catalyst does not always change colors. In summary, options 2, 3, and 4 are true of a catalyst, while options 1 and 5 are not.
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Regarding the options provided:
1) False - A catalyst is not used up during a reaction.
2) True - In the body, catalysts are often enzymes that facilitate biochemical reactions.
3) True - Catalysts change the rate of a reaction by providing an alternative pathway with a lower activation energy.
4) True - Catalysts affect the rate-limiting step, which is the slowest step in a reaction and determines the overall rate.
5) False - Catalysts do not always change colors, as their function is related to altering reaction rates rather than causing visual changes.
So, the true statements are 2, 3, and 4.
To know more about catalyst. please visit.....
brainly.com/question/31870054
#SPJ11
The the _____ temperature _____ the the air molecules move. (Choose 2) higher, faster lower, faster lower, slower higher, slower
Answers
Answer:
The higher the temperature the faster the air molecules move. / The lower the temperature the slower the air molecules move.
Explanation:
Both answers work. Higher temp = more energy = moving faster, and the opposite applies. Lower temp = less energy = moving slower.
Please helppp
Which of the following trends is decreasing as you move from left to right across this portion of the Periodic Table?
Atomic number
Metallic character (becoming less metal like)
Number of atomic orbitals
Number of valence electrons
Answers
Answer:
Metallic character (becoming less metal like)
Explanation:
I took the test

In which state of matter has the LEAST kinetic energy?
A gasgas
B liquidliquid
C solidsolid
D plasma
Answers
I think it's c I'm not sure
Need ASAP temperatures plummet with this type of weather front

Answers
the answer is cold front
A water tank holds 18,000 gallons. How long will it take for the water level to reach 6,000 gallons if the water is used at an average rate of 450 gallons per day?
Answers
18000 - 450d = 6000
18000 - 6000 = 12000
12000 / 450 ≈ 26.7
450 = 26.7 ≈ 12000
Good luck!
A football player runs in a straight line down the field, crossing the 20-yard line when the stopwatch reads 12 seconds and crossing the 70-yard line when the stopwatch reads 17 seconds. What is his speed in yards per second?
Answers
Answer: 10y/s
Explanation:
a 100 ml solution containing 0.100 m nahco3 is treated with excess acid, producing carbon dioxide which is released into the atmosphere. how much work (in j) is done by the co2 if this reaction occurs at 298k and 1 atmosphere of pressure?
Answers
If the reaction occurs at 298K and 1 atmosphere of pressure, the work done by the CO₂ is -24.8 Joules.
How to calculate work (in joule) is done by the CO₂ if the reaction occurs at 298K and 1atm of pressure?Work is the energy required to move something against a force. The energy of a system can change due to work and other forms of energy transfer such as heat. Gases do expansion or compression work with the equation W = - P * ΔV.
From the question given
molarity of (NaHCO₃) = 0.1m
Volume of NaHCO₃ (V) = 100ml * 10⁻³ L / 1 ml = 0.1L
T (temperature) = 298k
P (pressure) = 1atm
moles of NaHCO₃
= molarity * Volume in Liters
= 0.1m * 0.1L = 0.01L
NaHCO₃ + HCl -> CO₂ + H₂O + NaCl
moles of CO₂ = moles of NaHCO₃ * (coefficient of CO₂ / Coefficient of NaHCO₃)
= 0.01L * 1/1
= 0.01 mol (moles of CO₂)
so, initial moles of CO₂ = 0
final moles of CO₂ = 0.01mol (based from the calculation)
Δn 0.01 - 0 = 0.01mol
Now, let's use work formula (as mentioned above on explanation part)
Work = - P * ΔV
= - Δn * R *T
Where R = 8.314J / mol.K
= - ( 0.01mol * 8.314 J / mol*K * 298K)
= -24.8J
Therefore, the work done by the CO₂ on this 100ml solution is -24.8Joules.
Learn more about work https://brainly.com/question/16687948
#SPJ4
Iron (Fe) does NOT fit in the pattern in column 7.
Give a reason why.

Answers
Iron (Fe) does NOT fit in the pattern in column 7 because Iron is a metal.
What are the characteristics of Iron (Fe)?
1. Iron (Fe) is a silvery-gray metal that is very malleable and ductile.
2. It has a melting point of 1538 °C (2798 °F) and a boiling point of 2862 °C (5164 °F).
3. It is the most abundant element in the Earth's crust, making up about 5.6% of its mass.
4. It is magnetic, and it is an important component of steel and other alloys.
5. Iron is also an essential element for biological processes, and it is necessary for the formation of hemoglobin in red blood cells.
Iron is not an element in column 7 of the periodic table because it is a transition metal, which is found in columns 3-12. Column 7 contains the halogens (Group 17), which are non-metallic elements. Allow (iron) had different properties (to oxygen and sulfur) and it ignore electrons.
Therefore, Iron is a metal is the answer.
To learn more about Iron (Fe) from the link
https://brainly.com/question/28303739
#SPJ1
if anyone can sole I give big brain
___. CO(g) +. ___ H2(g) ---> ___ C8H18(l) +. ___ H2O
Answers
Answer:
. 8 CO(g) + 17 H2(g) 1 C8H18(l) + 8 H2O
Explanation:
URGENT:
5 milliscruples is how many pounds?
20 grains=1 scruple
3 scruples=1 dram
8 drams= 1 ounce
16 ounces= 1 pound
Answers
Answer: 13.02083 lbs
Explanation:


I need help on this question really bad

Answers
Answer:
Fungi, or D
Explanation:
Write the purpose for the investigation.
What sort of a graph would best show these results?

Answers
The purpose of this investigation was to determine the relationship between the concentration of acid and the time taken for magnesium to react completely.
The students tested various concentrations of acid by diluting the acid solution to different strengths and recorded the time taken for magnesium to react completely.
To display the results of this investigation, a line graph would be the most suitable option. The x-axis should represent the concentration of the acid, while the y-axis should represent the time taken for magnesium to react completely. Each concentration level can be plotted as a data point on the graph, with a line connecting them to show the trend in the relationship between the concentration of the acid and the time taken for magnesium to react completely.
To know more about concentration, visit:
https://brainly.com/question/10725862
#SPJ1
why must a ph meter be calibrated by using a solution with a known ph value
Answers
Answer:
A pH calibration is the process of adjusting your pH meter by measuring solutions of a known pH value. This is because the characteristics of your electrode will change over time and this needs to be compensated for. A calibration does this by matching your pH meter to the current characteristics of your pH sensor.
Explanation:
Part C
The tablets were a source of carbon dioxide. What can you conclude about the effect carbon dioxide has in the
atmosphere?
Answers
A significant heat-trapping gas, or greenhouse gas, called carbon dioxide (CO2) is produced through the production and burning of fossil fuels like coal, oil, and natural gas as well as during wildfires and other natural processes like volcanic eruptions.
Thus, The first graph displays atmospheric CO2 measurements made recently at Hawaii's Mauna Loa Observatory without accounting for seasonal or natural variations.
The air bubbles trapped in ice sheets and glaciers over Earth's last three glacial cycles are used in the second graph to depict CO2 levels during those periods.
Human activities have increased atmospheric CO2 by 50% since the start of the industrial era (in the 18th century), bringing it to 150% of its value in 1750.
Thus, A significant heat-trapping gas, or greenhouse gas, called carbon dioxide (CO2) is produced through the production and burning of fossil fuels like coal, oil, and natural gas as well as during wildfires and other natural processes like volcanic eruptions.
Learn more about Carbon dioxide, refer to the link:
https://brainly.com/question/3049557
#SPJ1
Which transfers thermal energy in the same way the Suns energy is transferred to Earth?
A. The boiling water
B. The burner flame
C. The hot candle
D. The rising steam
Answers
Answer: I think this one is the boiling water
sorry if wrong
Explanation:
OMG PLEASE HELP IM DOING SO WELL IN SCIENCE AND THIS IS GRADED AND MY PROFEESER WONT HELP
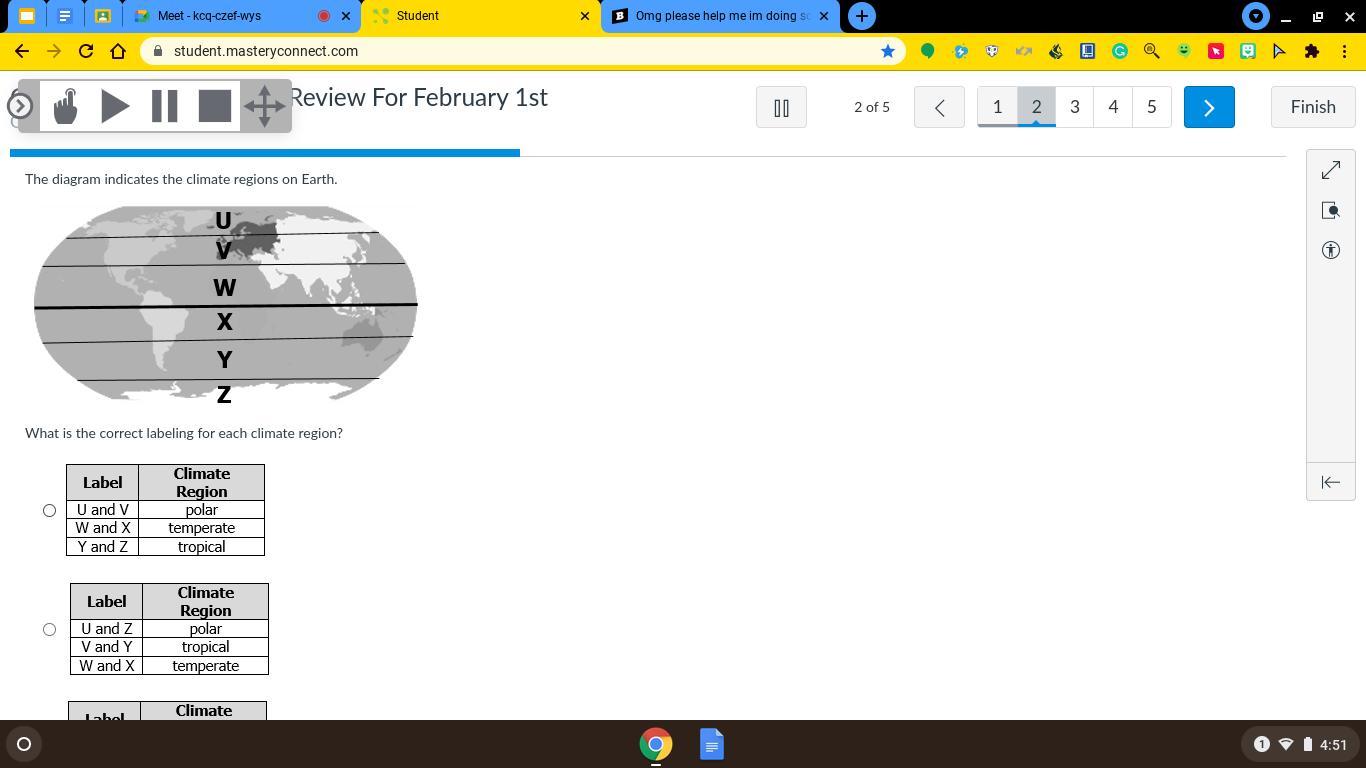

Answers
Answer:
bbbbbbbbbbbbbbbbbbb
Explanation:
a 1.16 mol sample of an element has a density of 13.55 g/cm3. if the sample occupies a volume of 17.17 cm3, what is the molar mass of the element?
Answers
To determine the molar mass of the element, we will use the formula:$$\mathrm{Molar\ mass}=\frac{\mathrm{Density}}{\mathrm{Moles}/\mathrm{Volume}}$$Given:$$\mathrm{Density}=13.55\:\mathrm{g/cm^3}$$$$\mathrm{Moles}=1.16\:\mathrm{mol}$$$$\mathrm{Volume}=17.17\:\mathrm{cm^3}$$.
Given that, density = 13.55 g/cm3molar mass = ?mass of sample = density x volume$$\therefore \ \text{mass of sample} = \mathrm{density} \times \mathrm{volume}$$$$\Rightarrow\mathrm{mass} =13.55 \:\mathrm{g/cm^3} \times 17.17 \:\mathrm{cm^3}=232.7315\:\mathrm{g}$$Now, Molar mass is given by,$$\mathrm{Molar\ mass}=\frac{\mathrm{Density}}{\mathrm{Moles}/\mathrm{Volume}}$$$$\Rightarrow\mathrm{Molar\ mass}=\frac{13.55\:\mathrm{g/cm^3}}{1.16\:\mathrm{mol}/17.17\:\mathrm{cm^3}}=103.1\:\mathrm{g/mol}$$Therefore, the molar mass of the element is 103.1 g/mol.
The given information is about the element's sample. The formula for molar mass is used to find out the unknown molar mass of the given element.
To know more about molar mass visit:
https://brainly.com/question/31545539
#SPJ11
What is the mass
notation number of an atom that has 9 protons, 11
neutrons, and 9 electrons?
Hisham Hassen. 8.06 AM
Due Tomorrow
Answers
Answer:
Didi you get it?we got the same class
Explanation:
The size of early universe was...
x a. same size as today
x b. the same size as the planet Earth
x c. medium sized
x d. smaller than an atom
Answers
Answer: A
Explanation:
The universe was infinite and is still infinite
Match the following terms to their units. A. Atomic mass B. Molarity C. Molar mass - mol/liter amu g/mol ne relationship between the atomic mass of an element and a mole point)
Answers
The matching is like :- (A) Atomic mass - amu (atomic mass units) (B) Molarity - mol/liter (C) Molar mass - g/mol (grams per mole)
The atomic mass (in amu) is used to convert between the mass of an element and the number of moles of that element, while the molar mass (in g/mol) is used to convert between the mass of a compound and the number of moles of that compound. Molarity (in mol/L) is used to express the concentration of a solution. In chemistry, the atomic mass of an element is the mass of a single atom of that element relative to the mass of a carbon-12 atom, which is defined as exactly 12 atomic mass units (amu). The atomic mass is typically given in units of amu, and it is used to convert between the mass of an element and the number of moles of that element. Molarity is a unit of concentration that is commonly used in chemistry. It is defined as the number of moles of solute per liter of solution. The unit for molarity is mol/L, which is often abbreviated as M. Molar mass is the mass of one mole of a substance. It is expressed in units of grams per mole (g/mol). The molar mass is used to convert between the mass of a compound and the number of moles of that compound. For example, if we know the atomic mass of an element (in amu), we can use it to calculate the molar mass of that element (in g/mol). Similarly, if we know the molarity of a solution (in mol/L) and the molar mass of the solute (in g/mol), we can calculate the mass of the solute in a given volume of the solution.
In summary, the atomic mass, molarity, and molar mass are all important concepts in chemistry that are expressed in different units. Understanding these units and how to use them is essential for many calculations in chemistry.
To know more about atomic mass please refer: https://brainly.com/question/17067547
#SPJ1
surfactants exert catalytic effects through which of the following mechanisms? A. Making the catalyzed reaction more energetically favorable
B. Changing the equilibrium constant of the catalyzed reaction to favor the products
C. Reducing the activation energy of the reaction
D. Covalently transferring a reactive functional group to a reactant
Answers
Surfactants reduce the activation energy of the reaction to exert catalytic effects, making the catalyzed reaction more energetically favorable. Option (C) is correct "Reducing the activation energy of the reaction".
Surfactants are chemical compounds that lower the surface tension between two liquids or between a liquid and a solid, which makes them useful in a wide range of industrial and domestic applications. They have the ability to exert catalytic effects through the reduction of activation energy of the catalyzed reaction.
Explanation:Surfactants are molecules that have both hydrophilic (water-loving) and hydrophobic (water-hating) ends. They reduce surface tension and interfacial tension between liquids and solids by adsorbing at the interface. In catalytic reactions, surfactants reduce the activation energy of the reaction by facilitating the movement of reactants from one phase to another or by stabilizing the transition state. This increases the rate of the reaction and decreases the activation energy. Thus, surfactants can act as effective catalysts for a variety of reactions.
Catalysis is the process of increasing the rate of a chemical reaction by adding a substance called a catalyst. A catalyst is a substance that increases the rate of a reaction without being consumed or permanently changed in the process. In a catalyzed reaction, the catalyst lowers the activation energy required for the reaction to occur. This allows the reaction to proceed at a faster rate, often with lower temperatures and pressures than would otherwise be required.
To know more about catalyzed reaction visit :
https://brainly.com/question/33366516
#SPJ11
what does the chemical formula Ba(HCO3)2 tell us about number of atoms of each element present
Answers
Answer: The chemical formula is Ch(HCO3)2 and Cn(HO3)2
Explanation:
There is 1 atom of Ba, 2 atoms of hydrogen, 2 atoms of carbon, and 6 atoms of oxygen.
What are molecules?The smallest particle of a substance has all of the physical and chemical properties of that substance.
The chemical formula Ba(HCO3)2 tells us about the number of atoms of each element present as follows:
1 atom of Ba
2 atoms of hydrogen
2 atoms of carbon
6 atoms of oxygen.
Learn more about molecules here:
brainly.com/question/14130817
#SPJ2
A 2.00L mixture of helium, nitrogen, and neon has a total pressure of 815 mmHg at a
temperature of 255K. If the partial pressure of helium is 201 mmHg and the partial
pressure of nitrogen is 351 mmHg, what is the partial pressure of neon in the mixture?
O 709 mmHg
O 512 mmHg
O 667 mmHg
O 263 mmHg
Answers
Answer: The partial pressure of neon in the mixture is 263 mm Hg
Explanation:
According to Dalton's law, the total pressure is the sum of individual pressures.
\(p_{total}=p_{He}+p_{N_2}+p_{Ne}\)
Given : \(p_{total}\) =total pressure of gases = 815 mm Hg
\(p_{He}\) = partial pressure of helium = 201 mm Hg
\(p_{N_2}\) = partial pressure of nitrogen = 351 mm Hg
\(p_{Ne}\) = partial pressure of Neon = ?
putting in the values we get:
\(815mm Hg=201 mm Hg+351 mm Hg+p_{Ne}\)
\(p_{Ne}=263mmHg\)
The partial pressure of neon in the mixture is 263 mm Hg
In a titration experiment 34.7 mL of a 0.145M solution of barium hydroxide [Ba(OH)2] is added to
20mL of hydrochloric acid (HCI) of unknown concentration until the equivalence point is reached.
What is a) the molarity of the acid? and b) How many grams of HIC are in the solution?
Answers
To determine the molarity of the hydrochloric acid (HCl) and the number of grams of HCl in the solution, we can use the principles of stoichiometry and the balanced chemical equation for the reaction between barium hydroxide (Ba(OH)2) and hydrochloric acid (HCl):
Ba(OH)2 + 2HCl → BaCl2 + 2H2O
a) Molarity of the acid (HCl):
From the balanced equation, we can see that one mole of Ba(OH)2 reacts with two moles of HCl. Therefore, the moles of HCl can be calculated as follows:
Moles of HCl = Moles of Ba(OH)2 = Molarity of Ba(OH)2 × Volume of Ba(OH)2 solution in liters
Moles of HCl = 0.145 M × (34.7 mL / 1000 mL/L) = 0.00504 moles
Since the volume of HCl is not specified, we cannot directly calculate the molarity of the acid. We need additional information, such as the volume of HCl used or the balanced equation stoichiometry.
b) Grams of HCl:
To calculate the grams of HCl, we need the molar mass of HCl. The molar mass of HCl is approximately 36.46 g/mol.
Grams of HCl = Moles of HCl × Molar mass of HCl
Grams of HCl = 0.00504 moles × 36.46 g/mol = 0.184 grams
Therefore,
a) The molarity of the hydrochloric acid (HCl) is not provided in the given information. Additional information is needed to calculate it.
b) The solution contains approximately 0.184 grams of HCl.
To know more about Molarity:
https://brainly.com/question/31545539
#SPJ1
Calculate the change in temperature of a 5.0 g sample of water that loses 1800J of heat energy.
Q = mcΔT
The specific heat of water is 4.18 J/g°C.
Answers
Taking into account the definition of calorimetry, the change in temperature of a 5.0 g sample of water that loses 1800J of heat energy is 86.12 C.
In first place, calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat is the amount of heat that a body can receive or give up without affecting its molecular structure. If the molecular structure does not change, the state (solid, liquid, gaseous) does not change. Since the molecular structure does not change, a change in temperature is observed.
In other words, sensible heat is the amount of heat that a body absorbs or releases without any changes in its physical state (phase change).
So, the equation that allows to calculate heat exchanges is:
Q = c× m× ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case, you know:
Q= 1800 J c= 4.18 \(\frac{J}{gC}\) m= 5 g ΔT= ?Replacing in the expression to calculate heat exchanges:
1800 J= 4.18\(\frac{J}{gC}\)× 5 g× ΔT
Solving:
1800 J ÷ (4.18\(\frac{J}{gC}\)× 5 g)= ΔT
ΔT= 86.12 C
In summary, the change in temperature of a 5.0 g sample of water that loses 1800J of heat energy is 86.12 C.
Learn more:
brainly.com/question/11586486?referrer=searchResults brainly.com/question/24724338?referrer=searchResultswhat is the first thing you do in any conversion question?
Answers
Answer:
You confirm the units involved, which would make you to understand the factors required.
Explanation:
Conversion of units is a process in which a given unit of a physical quantity is accurately transformed into another. Example, when the mass of a substance in grams is converted to kilograms. Or conversion of a given quantity of substance from liters to gallons.
When conversion is required, the first thing to do is to determine the units involved. That is, which unit do you want to convert to the required unit. This would give the understanding of the factors that are needed.