What is in catalytic converter that makes it valuable.
Answers
A catalytic converter contains valuable metals such as platinum, palladium, and rhodium. These metals are responsible for the catalytic reactions that reduce harmful pollutants emitted from an automobile's exhaust system into less harmful emissions.What is a catalytic converter?
A catalytic converter is a device found in the exhaust system of automobiles that is used to reduce the toxicity of pollutants emitted from the combustion of gasoline or diesel fuel in the engine. This device, which is often made of a ceramic honeycomb structure, is coated with valuable metals such as platinum, palladium, and rhodium. These metals act as catalysts, facilitating the chemical reactions that convert harmful pollutants such as carbon monoxide, nitrogen oxides, and unburned hydrocarbons into less harmful emissions such as carbon dioxide, nitrogen, and water vapor.What makes a catalytic converter valuable?The catalytic converter contains valuable metals such as platinum, palladium, and rhodium that are used in its construction. These metals are rare and expensive, with platinum being the most expensive of the three. As a result, catalytic converters are often stolen from vehicles and sold to scrap dealers for their precious metals content. Since these metals are in high demand in other industries such as jewelry and electronics, the theft of catalytic converters has become a lucrative business for thieves. To prevent theft, many vehicle owners and manufacturers have installed security devices or designed new types of catalytic converters that are less valuable to thieves.
For more information on catalytic converter visit:
brainly.com/question/15591051
#SPJ11
Related Questions
Physical or chemical change? Salt crystals forming on a hot day by the Great Salt Lake
Answers
The formation of salt crystals on a hot day by the Great Salt Lake is a physical change. This is because the process does not involve a chemical reaction that changes the composition of the salt. The heat simply causes the water to evaporate, leaving behind the salt crystals.
A physical property is a characteristic of a substance that can be observed or measured without changing the identity of the substance. Physical properties include color, density, hardness, and melting and boiling points. A chemical property describes the ability of a substance to undergo a specific chemical change.
#SPJ11
Learn more about physical and chemical changes on: https://brainly.com/question/11370755
Explain why mass is used to measure the properties of solids, liquids, and gases.
Answers
Answer: Mass takes into account the force of gravity.
Explanation:
g 50.0 g of ice at -22.0 are added to 120.0 g of water at 7.0 in an insulated container. (a) what will be the temperature when thermal equilibrium is reached? (b) how mass of ice will be present when equilibrium is reached?
Answers
a) The temperature of the system when thermal equilibrium is reached will be 0°C.
b) 30 g of ice will be present when thermal equilibrium is reached.
mass of ice (m1) = 50.0 g
Temperature of ice (T1) = -22.0°C
Mass of water (m2) = 120.0 g
Temperature of water (T2) = 7.0°C
The energy required to melt the ice is given by the equation,
Q1 = m1 × Lf
Where, Lf is the latent heat of fusion of ice = 334 J/g
Q1 = 50.0 × 334Q1 = 16700 J
The energy required to heat the ice from -22°C to 0°C (Q2) is given by,
Q2 = m1 × c × (0-(-22))
Where, c is the specific heat capacity of ice = 2.06 J/g°C
Q2 = 50.0 × 2.06 × 22Q2 = 2266 J
The energy lost by water (Q3) is given by the equation,
Q3 = m2 × c × (7 - 0)
Where, c is the specific heat capacity of water = 4.184 J/g°C
Q3 = 120 × 4.184 × 7Q3 = 35244.48 J
Total energy gained (Q4) by ice and water is equal to the energy lost by the water.
Q4 = Q1 + Q2
Q4 = 16700 + 2266
Q4 = 18966 J
18966 = Q3 = m2 × c × (7-0)
18966 = 120 × 4.184 × 7
m2 = 18966/(120 × 4.184 × 7)
m2 = 3.03 g
At equilibrium, the mass of the remaining ice (m3) can be calculated as follows,
Q1 + Q2 = m3 × Lf + m3 × c × (0 - 0°C)
16700 + 2266 = m3 × 334 + m3 × 2.06 × (0 - (-22))
m3 = 30 g
Therefore, the temperature of the system when thermal equilibrium is reached will be 0°C, and the mass of the ice remaining at equilibrium will be 30 g.
To learn more about "equilibrium", visit: https://brainly.com/question/517289
#SPJ11
Which of these choices describes a solid substance?
A. The volume of the substance depends on the container its in.
B. Volume of the substance changes depending on its location.
C. The shape of the substance changes often.
D. The shape of the substance is fixed and volume is constant.
Answers
liquids take the shape of the container
Solids are always fixed with a constant volume
Gases move around somewhat like a liquid
considering the ideal gas law, evaluate the following statement:for a given set of values for an ideal gas, where the number of moles and the pressure remain constant, if the volume increases what will happen to the temperature?
Answers
The volume increases to be the temperature rises, as well as decreases to be the temperature decreases.
These examples of a consequence of temperature on the volume for a given amount of a confined gas at constant stress are true in general: The volume increases to be the temperature rises, as well as decreases to be the temperature decreases. If the temperature measured in kelvin, volume as well as temperature remain directly proportional.
To know more about temperature, here:
https://brainly.com/question/7510619
#SPJ1
An acetaminophen suspension for infants contains 80 mg/0.80 mL suspension. The recommended dose is 15 mg/ kg body weight. How many mL of th is suspension should be given to an in fant weighing 14 lb? (Assume two significant figures.)
Answers
Answer:
the answer is 35mg and 58ml
Which of the following types of mass movement is LEAST coherent (most like a fluid)?
a. slump
c. rock slide
b. creep
d. mudflow
Answers
The type of mass movement that is LEAST coherent (most like a fluid) is a mudflow. The correct option is d.
Mass movement refers to the downhill movement of earth materials due to gravity. There are different types of mass movement, including slump, rockslide, creep, and mudflow. The coherency of a mass movement refers to the degree of internal strength or viscosity of the material involved.
The more coherent the material, the less it flows like a fluid. Among the given options, mudflow is the least coherent or most fluid-like type of mass movement. Mudflow refers to the rapid downhill movement of a mixture of water and fine-grained sediment, such as clay and silt.
Mudflows are highly fluid and can travel at high speeds, posing a significant hazard to life and property in areas prone to landslides and flash floods. In contrast, slumps, rockslides, and creep involve more cohesive materials and exhibit less fluid-like behavior. Therefore, the correct option is d.
To know more about mudflow refer here:
https://brainly.com/question/30922995#
#SPJ11
What is nuclear energy plants?
Answers
The following properties are either physical or chemical. Which one is different from the rest based on those two categories? (5 points)
Boiling point
Density
Ductility
Heat of combustion
Answers
Answer:
ductility
Explanation:
It is a mechanical property
Which of the following types of energy does an object store in the bonds of atoms and molecules? (5 points)
Оа
Chemical energy
Ob
Gravitational energy
Ос
Kinetic energy
Od
Sound energy
Answers
Answer:
sound energy
Explanation:
Answer:
Chemical energy
Explanation:
what is your definition of science?
**ANSWER AND ILL GIVE YOU BRAINLIEST ANSWER**
Answers
If 43.6J of heat is added to a 0.985g block of metal, the temperature of the metal increases by 12.4C. Calculate the specific heat capacity of the metal.
Answers
The specific heat capacity of the metal when the temperature rises by 12.4°C is 3.569 J/g°C.
What is specific heat capacity?Specific heat capacity is defined as the heat capacity of the substance divided by the mass of the substance.It is the amount of heat added to one unit mass of substance to increase it's temperature by one unit.The SI unit is joule per kelvin per kilogram.
It varies with temperature and for each different state of matter it is different.Specific heat capacity of a substance is higher when it is allowed to expand as a result of heating.It is measured by a technique called differential scanning calorimetry.
It is calculated by the given formula,
W=mcΔt
where W=energy supplied
m= mass of substance
c=specific heat capacity
Δt= temperature change
Substituting the values given in the problem in the above equation,
c=43.6/0.985×12.4=3.569 J/g°C
Thus, the specific heat capacity of metal is 3.569 J/g°C.
Learn more about specific heat capacity ,here:
https://brainly.com/question/28302909
#SPJ1
What does the heliocentric model of the solar system state?
Answers
Heliocentric model of the solar system state the sun is assumed to lie at or near a central point and the earth and other bodies revolve around it.
Heliocentrism is the astronomical model developed by Nicolaus Copernicus and published in 1543 and this model postulate that the sun at the center of the universe with Earth and the other planets orbiting around it in circular paths and main heliocentric means earth revolve around the sun is called as heliocentric theory
Know more about heliocentric
https://brainly.com/question/18403954
#SPJ1
can someone answer pls

Answers
Students conducted an analysis of baking soda in chemistry lab. During the activity, they had to calculate the mass in
grams of the baking soda being used. Some student data appears in the table. At the end of the activity, the teacher
told the students that the actual mass of baking soda was 0.45 grams. Which student had the most accurate and
precise data?
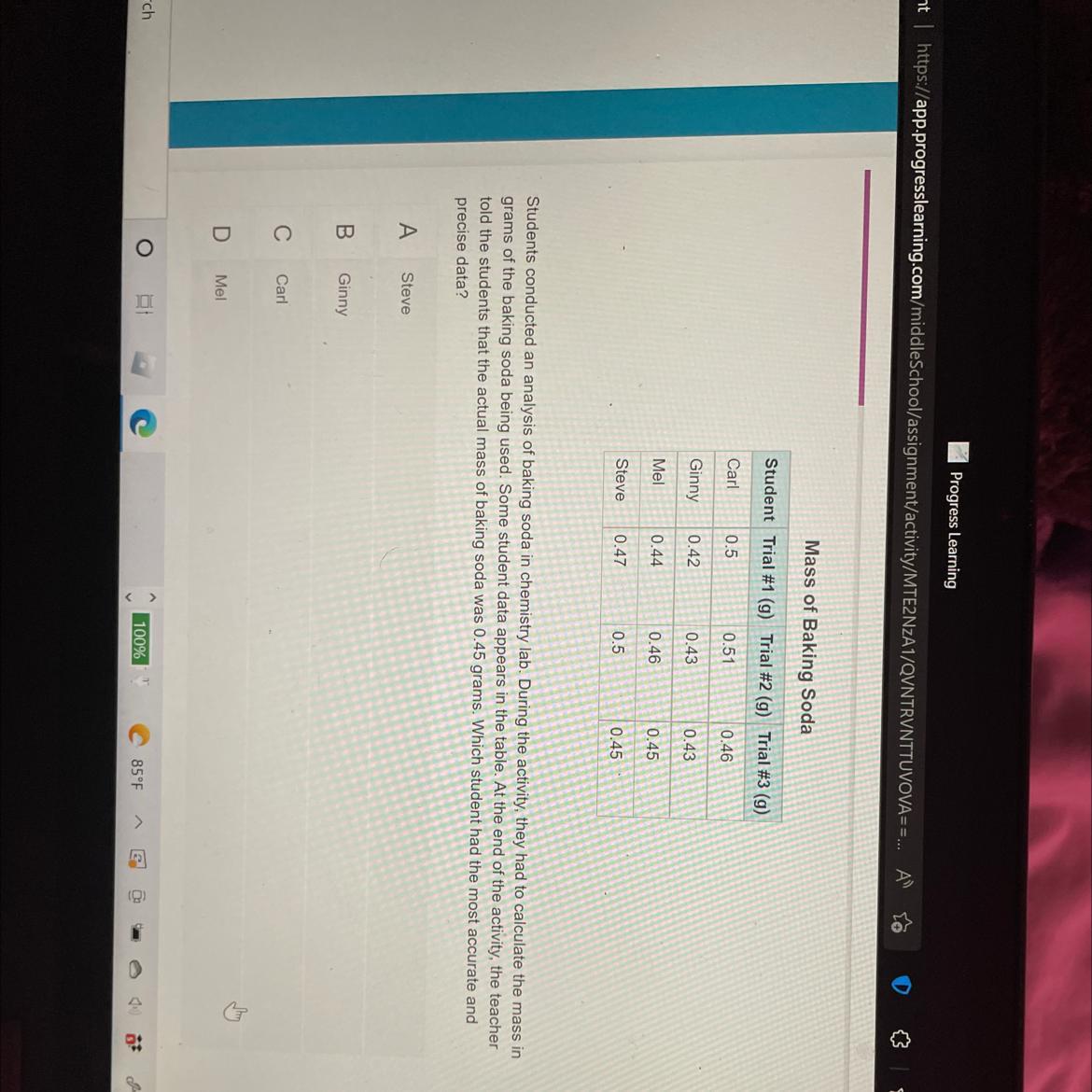
Answers
The student that had the most accurate and precise data is Mel (option D).
What is accuracy and precision?Accuracy is the degree of conformity of a measure to a true or standard value i.e. closeness to a standard or true value.
On the other hand, precision is the ability of a measurement to be reproduced consistently i.e. the closeness of measured values.
According to this question, students calculated the mass in grams of the baking soda being used.
It can be said that Mel, which measured the following values: 0.44g, 0.46g, 0.45g had the most accurate and precise value because they are both close to themselves and the true value.
Learn more about accuracy and precision at: https://brainly.com/question/15276983
#SPJ1
Answer:
D
Explanation:
Mel
4. Manik saw his father watering his garden plants in hot weather. He noticed that
water doesn’t stick to the plant leaves and leaves become dry but looked fresh. He asked
following questions to his teacher
a. Which tissue forms the outer covering of a plant and does it have a protective role
to play?How ?
b. Why does water not stick to the leaves?
Answers
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
What tissues protects the leaves?We know that the leaves are the parts of the plant that are involved in photosynthesis. Photosynthesis is the process by which green plants produce their own food in the presence of sunlight and chlorophyll. We know that the leave has an outer protective covering.
The tissue that plays this outer covering of a plant for is the epidermis and its waxy cuticle. It prevents damage to the plant.
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
Learn more about leaves:https://brainly.com/question/12539285
#SPJ1
What is the notation for the enthalpy of solution?
O -Hsol
O AH sol
Ο ΔΕ
O +Hsol
Answers
The notation for the enthalpy of the solution is ∆Hsol. The correct answer is option ∆Hsol.
The enthalpy of solution is a measure of the amount of heat absorbed or released when a solute is dissolved in a solvent to form a solution. If the value of ∆Hsol is positive, it means that heat is absorbed during the process of dissolving the solute, while a negative value of ∆Hsol indicates that heat is released during the same process. This value is often used to predict whether a given solute will dissolve in a given solvent, as well as the relative amounts of solute and solvent that will be required to form a solution. The enthalpy of solution can be calculated experimentally by measuring the temperature change that occurs when a known amount of solute is dissolved in a known amount of solvent. Alternatively, it can be calculated theoretically using thermodynamic data for the solute and solvent.For more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
Explain how balancing chemical equations relates to the law of conservation of matter.
Answers
Answer:
In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Showing that Mass cannot be created nor destroyed in a chemical reaction
Explanation:
Explain the volume of ideal gasses as related to the molar concept.
Answers
Answer:
n = Initial volume/22.4L
Explanation:
The molar concept is simply one that is used to find the Number of moles and explain the relationship it has with avogadro's number, molecular mass, molar mass e.t.c.
Now, in terms of molar mass, number of moles is given by the formula;
n = mass of the sample/molar mass
In terms of avogadro's number, number of moles is;
1 mole = avogadro's number = 6.02 × 10^(23)
Now, when dealing with ideal gases, the molar volume of an ideal gas is 22.4 L.
Now the relationship between this volume and the mole concept is that the number of moles is gotten by dividing the initial volume by this molar volume.
Thus;
n = Initial volume/22.4L
How much energy does a wave with a frequency of 7.0 x 1012 Hz have
Answers
Answer:
4.64 × 10⁻²¹ J/s
Explanation:
[Only if the wave travels at the speed of light]
Because a wave presumed to be at light speed is a photon, E = hf.
If frequency is not given, E = hc/λ
c is the speed of light in meters per second. c = 299, 792, 458 m/s.
λ is the wavelength which is dependent.
h or plancks constant = 6.62607004 × 10⁻³² m² kg / s.
E is in units of J / s or jules ( m² kg ) per second.
If the wave has a frequency of 7.0 x 10¹² Hz or 7 THz (Tera-Hertz).
E = (6.62607004 × 10⁻³⁴) × (7.0 × 10¹²) =
46.38249028 × 10⁻²² =
4.638249028 × 10⁻²¹ J/s ≈
4.64 × 10⁻²¹ J/s
Why are the oxidation and reduction half-reactions separated in an
electrochemical cell?
Answers
Answer:
Electrochemical cells typically consist of two half-cells. The half-cells separate the oxidation half-reaction from the reduction half-reaction and make it possible for current to flow through an external wire.
Explanation:
The air pressure above a large body of water off the coast of Florida is much higher compared to the air above the coast. What direction would the wind blow in this situation? (i know its kinda easy but im dum please help)
A
from the ocean to the coast
B
from the coast to the ocean
C
from the ocean, vertically into the sky
D
from the coast, vertically into the sky
Answers
The direction in which the wind will blow in this situation is from the ocean to the coast and is denoted as option A.
What is Pressure?
This is defined as the force per unit area of a body and the unit is Pascal. We were told that air pressure above a large body of water is higher than the air above the coast.
This will make the wind blow from the ocean to the coast as the ocean has a higher pressure.
Read more about Pressure here https://brainly.com/question/25736513
#SPJ2
What is the molecular weight of the product of the reaction between anthracene and maleic anhydride given that diels-alder reactions have 100% atom economy? answer with just the number for the mass to two decimal places in g/mol.
Answers
276.44 is the molecular weight of the product of the reaction between anthracene and maleic anhydride given that Diels-alder reactions have 100% atom economy.
What occurs in the Diels-Alder reaction between maleic anhydride and anthracene?In this assignment, you will learn how to create a bridging polycyclic anhydride using the Diels-Alder method. Maleic anhydride and anthracene are two compounds that will dissolve in xylene and reflux. Utilizing vacuum filtering, the product 9,10-dihydroanthracene-9,10-succinic anhydride will be recovered and separated.
What is the name of the anthracene and maleic anhydride reaction?Anthracene and maleic anhydride in the Diels-Alder process.
To know more about Diels-alder reaction visit:
https://brainly.com/question/28258289
#SPJ4
3) Examine the list of molecules below. Place an X on the line next.

Answers
Compounds are made up of two or more bound elements.
Here, H2O, CO2, CH4 are Compounds.
How are molecules and compounds different from elements and mixtures?Students must be able to distinguish between molecules, compounds, elements, and mixtures by understanding very small features. A substance called a molecule is made up of two or more atoms that are joined together. such as oxygen O2. Compounds are made up of two or more bound elements, such the NaCl in table salt. Elements are substances that are unbreakable, pure, and have the same number of protons in their nuclei, such as gold Au, oxygen O, and hydrogen H. Mixtures are composed of two or more different substances but do not chemically link together.To learn more about : Molecules
Ref : https://brainly.com/question/26044300
#SPJ13
Wilkinson's catalyst accomplishes which of the listed molecular syntheses?O syn addition of H2 to an alkene O anti addition of H2 to an alkene O syn dihydroxylation an alkene O anti dihydroxylation an alkene
Answers
In particular, it accomplishes the: anti-addition of H2 to an alkene, meaning that the hydrogen atoms are added to opposite sides of the double bond. This reaction is called the Wilkinson hydrogenation.
Wilkinson's catalyst is a transition metal complex used in homogeneous catalysis. It is a rhodium complex, commonly used to catalyze the hydrogenation of alkenes.
The reaction is initiated by coordination of the alkene to the rhodium complex. The complex then undergoes oxidative addition of dihydrogen, producing a hydride complex. The hydride complex adds to the coordinated alkene, producing a rhodium alkyl complex.
The final step is reductive elimination of the alkane and the regenerated rhodium complex. The overall result is the addition of two hydrogen atoms to the alkene, anti to each other.
The other listed syntheses, such as syn addition of H2 to an alkene or dihydroxylation, are achieved through different reaction mechanisms and different catalysts.
To know more about "Anti-addition" refer here:
https://brainly.com/question/14078347#
#SPJ11
31. Use the equation given below to answer the following question: If 755 kJ of heat was absorbed, what mass of carbon dioxide reacted?
2CO2 + 43.9 kJ → 2CO +O2
Answers
Answer:
1514g of CO₂ reacted
Explanation:
Based on the reaction:
2CO₂ + 43.9kJ → 2CO + O₂
2 moles of carbon dioxide require 43.9kJ of energy to produce 2 moles of carbon monoxide and 1 mole of oxygen
To solve this question, we must convert the 755kJ of energy to moles of carbon dioxide that reacts and to find the mass as follows:
755kJ * (2 moles CO₂ / 43.9kJ) =34.4 moles of CO₂ are produced
Mass CO₂: Molar mass: 44.01g/mol
34.4 moles CO₂ * (44.01g / mol) =
1514g of CO₂ reactedWhat is (are) the products(s) of the following reaction?
Na + Cl --> NaCl
Group of answer choices
Cl
Na and Cl
NaCl
Na
Answers
Answer:
Na + Cl is a synthesis reaction so their product would only just be NaCl
Also, chlorine is a diatomic molecule it should be Na + Cl2 ---> NaCl
Balanced equation:
2Na + Cl2 -----> 2NaCl
What element should I do a project on?
Answers
Answer:
Project scope statement?
Explanation:
it's good one just do research on it
Nickel has a cubic unit cell. The edge of the unit cell is 3.524
x 10^(-8)cm. Determine the atomic radius of Nickel.
Answers
The approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
In a cubic unit cell, the body diagonal length (diagonal that passes through the center of the unit cell) is equal to four times the atomic radius (4r). We can use this relationship to find the atomic radius of nickel.
Given: Edge length of the unit cell (a) = 3.524 × 10^(-8) cm
The body diagonal length is given by:
Diagonal length (d) = a√3
Substituting the given values:
d = (3.524 × 10^(-8) cm) × √3
Now, we can calculate the atomic radius (r) by dividing the diagonal length by 4:
r = d / 4
Performing the calculations:
r = [(3.524 × 10^(-8) cm) × √3] / 4
r ≈ (3.524 × 10^(-8) cm) × (1.732 / 4)
r ≈ 1.532 × 10^(-8) cm
Therefore, the approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
Know more about atomic radius here
https://brainly.com/question/18095927#
#SPJ11
HELP PLEASE
Based on the organization of the Periodic Table of the Elements, what kind of bond does
chlorine form with both sodium and potassium?
A)ionic
B)metallic
© covalent
D)hydrogen
Answers
a) ionic
chlorine is present in top of group 17 and right of period 3 and
electronegativity(the tendency to gain electrons)
increases up the group and right the group.
so it has high tendency to form anion.
In the same way both Na and K are present up the group and left side and the tendency to lose electrons in both becomes high and they tend to form cation.