We mixed 30 ml of 1.0 M HCl with 70 ml of 1.0 M NaOH. What is the theoretical value for the temperature increase? Express your answer in ∘C.
a) 0.0 ∘C
b) 10.0 ∘C
c) 20.0 ∘C
d) 30.0 ∘C
Answers
Let's determine the theoretical value for temperature increase. Option (a) is correct. The temperature change (∆T) is calculated by using the following formula:
∆T = q / m * C
where, q = heat, m = mass and C = specific heat capacity.So, we can say that:
Theoretical value of temperature increase = ∆TWe
know that:Concentration of HCl (C1) = 1.0 MConcentration of
NaOH (C2) = 1.0 MVolume of HCl (V1) = 30 ml
Volume of NaOH (V2) = 70 ml
Molar mass of HCl = 36.5 g/mol
Molar mass of NaOH = 40 g/molDensity of HCl = 1.18 g/ml
Density of NaOH = 1.25 g/ml
Specific heat of the mixture (Cp) = 4.18 J/g °C
Since, the given HCl and NaOH solutions are present in equal amounts, so their molarity and density will be the same. Now, let's find out the mass of HCl and NaOH we have taken:Mass of HCl = Volume × Density = 30 ml × 1.18 g/ml = 35.4 g Mass of NaOH = Volume × Density = 70 ml × 1.25 g/ml = 87.5 gNow, let's calculate the heat evolved in this reaction: Heat evolved (q) = m × C × ∆T, where q = 0, m = 123 g (total mass of the solution) and C = 4.18 J/g °C.Then,∆T = 0 / (123 g) × 4.18 J/g°C ∆T = 0. So, the theoretical value for the temperature increase is 0.0 °C.
To know more about temperature visit:-
https://brainly.com/question/11464844
#SPJ11
Related Questions
wel yiald a total of 65,000 gallons of lacquer thinner that can be sold for $9. to a gation. The 2. Should Casidio sol tha acedone as is or process it into laccpor thrreer? 3how the adestional processing will cost 50.60 per gation of lacquer thinner, To sell the lacoguet thinner, Castlla Clyarical must pay shipping of 50.19 a galion and atministrative expenses of 50.13 a gallon on the thinher Requirement 1. Identity the surk cost ta the sunk cost reievant to Casillos decision? Why or why not? Castillo Chemical has spent $242,000 to refine 74,000 gallons of acetone, which can be sold for \$1.90 a gallon. Alternatively, Castillo Chemical can process the acetone further. This processing will yield a total of 65,000 gallons of lacquer thinner that can be sold for $3.10 a gallon. The additional processing will cost $0.60 per gallon of lacquer thinner. To sell the lacquer thinner, Castillo Chemical must pay shipping of $0.19 a gallon and administrative expenses of $0.13 a gallon on the thinner. Requirements 1. Identify the sunk cost. Is the sunk cost relevant to Castillo's decision? Why or why not? 2. Should Castillo sell the acetone as is or process it into lacquer thinner? Show the expected net revenue difference between the two alternatives.
Answers
1. Sunk Cost: The $242,000 spent on refining the acetone is a sunk cost and not relevant to future decisions.
2. Decision Analysis: Processing the acetone into lacquer thinner yields higher expected net revenue ($1,100 more) than selling it as is. Therefore, Castillo Chemical should choose to process the acetone into lacquer thinner.
1. Sunk Cost:
The sunk cost in this scenario is the $242,000 spent on refining the 74,000 gallons of acetone. A sunk cost is a cost that has already been incurred and cannot be recovered, regardless of the decision taken. It is not relevant to Castillo's decision on whether to sell the acetone as is or process it into lacquer thinner. The reason is that the sunk cost is in the past and should not influence future decisions. It cannot be changed or avoided, regardless of the course of action chosen.
2. Decision Analysis:
To determine whether Castillo Chemical should sell the acetone as is or process it into lacquer thinner, we need to compare the expected net revenue from both alternatives.
Option 1: Sell Acetone as is
Revenue from selling 74,000 gallons of acetone at $1.90/gallon = 74,000 gallons * $1.90/gallon = $140,600
Option 2: Process Acetone into Lacquer Thinner
Total revenue from selling 65,000 gallons of lacquer thinner at $3.10/gallon = 65,000 gallons * $3.10/gallon = $201,500
Total Cost of Processing:
Processing cost = 65,000 gallons * $0.60/gallon = $39,000
Shipping cost = 65,000 gallons * $0.19/gallon = $12,350
Administrative expenses = 65,000 gallons * $0.13/gallon = $8,450
Total Cost of Processing = $39,000 + $12,350 + $8,450 = $59,800
Net Revenue Difference:
Net revenue from processing = Total revenue - Total Cost of Processing
Net revenue from processing = $201,500 - $59,800 = $141,700
Expected Net Revenue Difference:
Expected Net Revenue Difference = Net revenue from processing - Revenue from selling acetone as is
Expected Net Revenue Difference = $141,700 - $140,600 = $1,100
The expected net revenue difference between selling the acetone as is and processing it into lacquer thinner is $1,100 in favor of processing the acetone. Therefore, based on the expected net revenue, Castillo Chemical should choose to process the acetone further into lacquer thinner, as it results in higher expected profitability compared to selling the acetone as is.
Learn more about revenue from given link
https://brainly.com/question/29786149
#SPJ11
mention the three colours of Zanzibar .
Answers
Answer:
horizontal tricolor with three equal-sized stripes of blue, black and green.
Explanation:
hope this is what u want

Answer: ↓
Black and Vivid Cerulean,
About The Photos: This second Photo is the flag from the 2000's I believe, and the first Photo shows the 1964 flag.

Calculate the pH and pOH of a solution that has a hydroxide ion concentration of 1.0 x 10^5. Acid or Base?
Answers
pOH = -log[OH-]
where [OH-] is the concentration of hydroxide ions in moles per liter.
Substituting the given value, we get:
pOH = -log(1.0 x 10^5)
pOH = -5
To find the pH of the solution, we can use the fact that pH + pOH = 14 (for a neutral solution at 25°C). Rearranging this equation, we get:
pH = 14 - pOH
Substituting the pOH value we just found, we get:
pH = 14 - (-5)
pH = 19
Since the pH of the solution is greater than 14, which is not possible for an aqueous solution at 25°C, it means that the given hydroxide ion concentration of 1.0 x 10^5 is not physically possible. Therefore, we cannot determine whether the solution is an acid or a base based on this information.
In what circumstance will a flash of lightning travel through the sky and touch the ground?
Answers
A metal block has a mass of 15 grams and when it is dropped in a graduated cylinder with water, the rise in volume is shown in the picture. (which is same as the volume of the metal block, or the volume of the metal block is equal to the difference in volumes in both pictures) What is the density of metal block in g/mL

Answers
Answer:
Depending on your answer choices it should be 1.33. I hope this helps didn't really know how to explain it.
Explanation:
Old photographic flashbulbs burn magnesium metal in a reaction that causes a flash of brilliant white light. A photographer would need to take care not to suffer burned fingers when trying to replace the hot bulb. What best identifies the energy transformation that occurs in the flashbulb? chemical energy to electromagnetic energy and thermal energy electromagnetic energy to chemical energy and thermal energy chemical energy to electromagnetic energy thermal energy to chemical energy
Answers
Answer:
A
Explanation:
Answer:
A
Explanation:
Not D or B
Common Mechanism Steps
•A_________________ is an electron-rich species that can donate a pair of electrons to form a new covalent bond.
Answers
A nucleophile is an electron-rich species that can donate a pair of electrons to form a new covalent bond. In organic chemistry, nucleophiles play an essential role in many chemical reactions, particularly in substitution and addition reactions.
Nucleophiles can be either neutral molecules, such as water, ammonia, or alcohols, or negatively charged species, such as anions or carbanions.
The reaction mechanism involving nucleophiles typically proceeds through several steps. The first step is the attack of the nucleophile on the electrophilic site of the substrate molecule. The electrophilic site is typically an atom with a partial positive charge, such as a carbon atom in a carbonyl group or a halogen atom in a halogenated alkane.
After the nucleophile attacks the electrophilic site, a new covalent bond is formed between the nucleophile and the substrate molecule. This results in the formation of an intermediate species that is usually unstable and highly reactive.
In the final step, the intermediate species is either transformed into the final product or regenerated to its original form by the loss of a leaving group. The leaving group is typically a weakly basic group, such as a halide ion or a water molecule.
Learn more about nucleophile here:
https://brainly.com/question/10702424
#SPJ11
what is the spectator ion in this reaction: lino3(aq) na (aq) -> nano3(s) li (aq)
Answers
In the given reaction, the spectator ion is NO₃⁻ (nitrate ion).
A spectator ion is an ion that does not participate in the overall chemical reaction and remains unchanged throughout the reaction. It is present on both sides of the equation.
In this case, LiNO₃ and NaNO₃ are both soluble compounds that dissociate into their respective ions in aqueous solution. The Li⁺ (lithium ion) and Na⁺ (sodium ion) are involved in the reaction, but they are not spectator ions.
On the other hand, NO₃⁻ appears as a common ion in both the reactant (LiNO₃) and the product (NaNO₃). It does not undergo any chemical changes and remains in solution as a spectator ion.
Therefore, the spectator ion in this reaction is NO₃⁻.
To know more about spectator ion, refer here:
https://brainly.com/question/28334213#
#SPJ11
Ammonia (NH3) decomposes to hydrogen ( H2) and nitrogen (N2) and 22.0 kcal/mol of energy was
absorbed as shown in the balanced equation below:
2 NH3(g) 3H2(g) + N2 (g) ΔH = + 22.0 kcal/mole
List the conversion factors for this reaction.
a. How many kilocalories of energy will be absorbed if 15.5 moles of ammonia (NH3) was decomposed?
b. How much energy is absorbed when 250.5 grams of hydrogen H2) are produced?
c. How much energy will be absorbed in order to produce 1530.0 grams of nit
d. How many grams of ammonia (NH3) should be decomposed in order to release 5500 kilocalories of energy?
Answers
To release 5500 kilocalories of energy, approximately 5500 grams of NH₃ should be decomposed.
The conversion factors for the given reaction are:
1 mole of NH₃ produces 3 moles of H₂ and 1 mole of N₂.
ΔH = + 22.0 kcal/mol (energy absorbed per mole of NH₃ decomposed).
a. To calculate the kilocalories of energy absorbed when 15.5 moles of NH₃ is decomposed:
Energy absorbed = ΔH * moles of NH₃
Energy absorbed = 22.0 kcal/mol * 15.5 mol = 341.0 kcal
b. To determine the energy absorbed when 250.5 grams of H₂ are produced, we need to convert the mass of H₂ to moles using its molar mass:
Molar mass of H₂ = 2.02 g/mol
Moles of H₂ = Mass / Molar mass = 250.5 g / 2.02 g/mol = 124.0 mol (approximately)
Energy absorbed = ΔH * moles of NH₃
Energy absorbed = 22.0 kcal/mol * 124.0 mol = 2,728.0 kcal
c. To find the energy absorbed in order to produce 1530.0 grams of N₂, we need to convert the mass of N₂ to moles using its molar mass:
Molar mass of N₂ = 28.02 g/mol
Moles of N₂ = Mass / Molar mass = 1530.0 g / 28.02 g/mol = 54.68 mol (approximately)
Energy absorbed = ΔH * moles of NH₃
Energy absorbed = 22.0 kcal/mol * 54.68 mol = 1,202.96 kcal (approximately)
d. The grams of NH₃ needed to release 5500 kilocalories of energy, we can rearrange the equation:
Grams of NH₃ = Energy released / (ΔH * moles of NH₃)
Grams of NH₃ = 5500 kcal / (22.0 kcal/mol * 1 mol)
Grams of NH₃ = 5500 g (since 1 kcal = 1 g)
To learn more about kilocalories refer here:
https://brainly.com/question/30667858#
#SPJ11
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 amu (85.00 % abundance) and 33.96
amu (15.00% abundance)
Answers
Explanation:
hope the picture helps you to understand:)
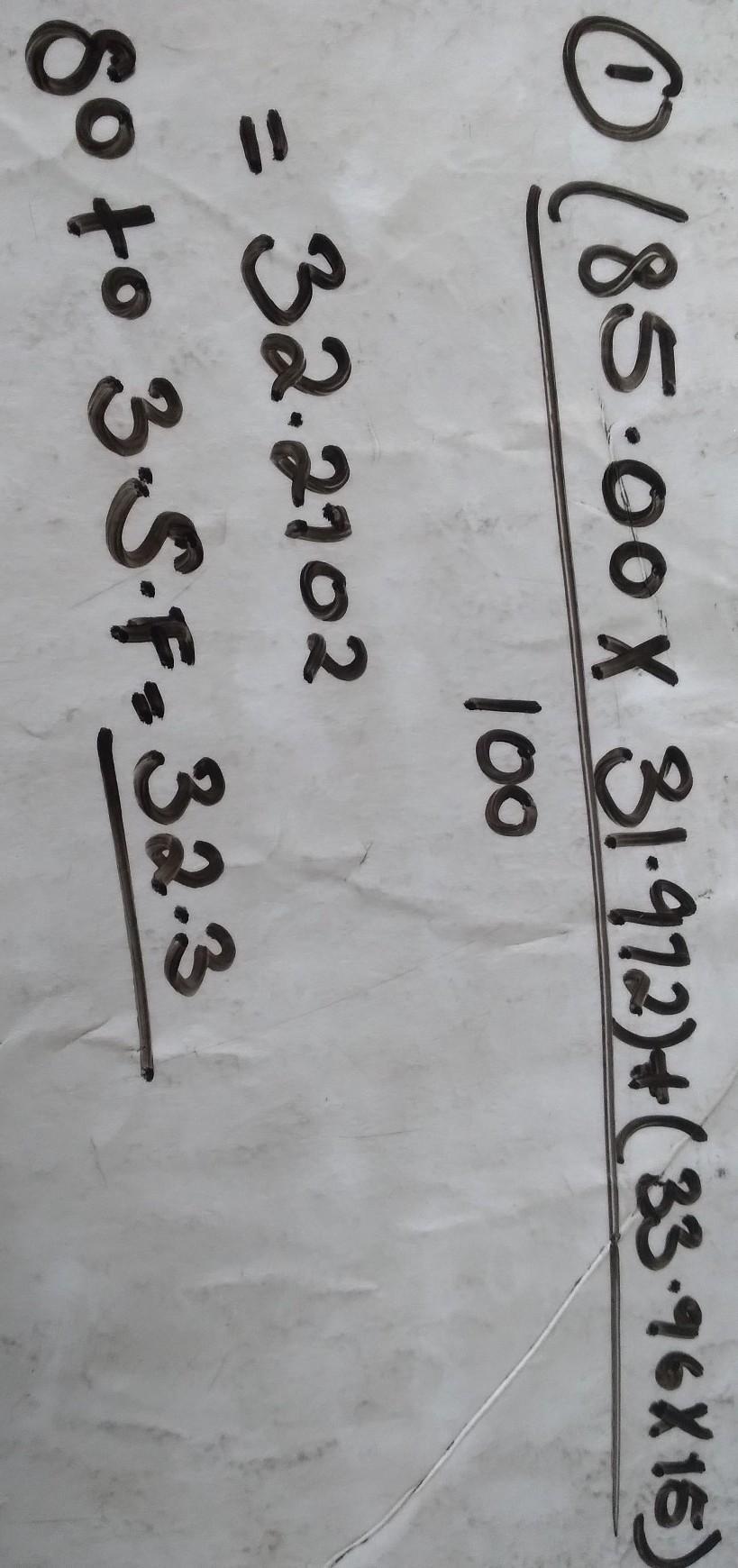
what is the standard form of 12,341 us?
Answers
Answer:
12341 in standard form = 1.2341 × 10⁴
Explanation:
When you split the number 12341 into a coefficient and a power of 10 you do get 12341 in exponential form, but there is an indefinite number of possibilities.
As you probably want 12341 in normalized scientific notation, the coefficient or significand of 12341 in scientific notation must be in the interval [1,10[.
As there are many ways to express 12341 in scientific notation, in this post we mean 12341 in normalized scientific notation, unless stated otherwise. Therefore:
12341 in scientific notation = 1.2341 × 10⁴.
This can also be expressed as 1.2341 × 10⁴, using the caret symbol, or as 1.2341e+4, which is called 12341 in e-notation.
What is the mass of the computer if the force is 15 N and the acceleration is 3
m/s2
Answers
Answer:
m=5k.gExplanation:
F=m×a sooo m=f÷am=15k.g.mls^2÷3m/s^2m=5k.g1. Which of the following is not among the first twenty elements? A. Chlorine B. Beryllium C. Iron D. Calcium 2. The number of atoms in one mole of an element is referred to as A. molecularity B. chemical symbol C. atomicity D. chemical element 3. What is the chemical symbol of Aluminum? A. AL B. Al C. aL D. al. 4. Which of the following is a heterogeneous mixture? A. Soft drink B. Air C. Flood C. Solution salt 5. Which of the following is false about mixtures? A. They can easily be separated by Chemical method B. They are not represented by chemical formula C. They can be homogenous or heterogeneous D. The constituents are physically combined 6. Which of the following elements is diatomic? A. Sodium B. Potassium C. Helium D. Oxygen 7. A diatomic element has ___________ number of atoms in one molecule of its element. A. one. B. two. C. three. D. four. 8. Which of the following is an example of a colloidal solution? A. Chalk in water B. Sand in water C. Sugar solution D. Solution of eggwhite 9. A mixture whose constituents are uniformly mixed is referred to as a A. homogeneous mixture B. suspension C. heterogeneous mixture D. colloidal solution 10. _____________ is a force that opposes motion when two surfaces are in contact with each other. A. Movement B. Pressure C. Friction D. gravity 11. _____________ is a substance, which cannot be split or broken down into any simpler form by an ordinary chemical process. A. Mixture B. Element C. Compound D. Metals. 12. Alloys are best classified as A. elements B. compounds C. mixtures D. non-metals 13. A type of force which occurs when a body moves constantly over another body is the _______ A. static force B. dynamic force C. magnetic force D. frictional force. 14. Which of the following is NOT a characteristic of friction? A. The ratio of the frictional force and the normal reaction is constant. B. The frictional force is always equal to the force applied through weight. C. Static frictional force is independent of the surface area in contact. D. Friction supports motion. 15. Use of ball or roller bearing on a turning wheel of a machine A. can cause wear and tear of the wheel B. reduces friction between the wheel and the body parts of the machine C. decreases the efficiency of the machine. D. hinders the productivity of the machine. 16. Another way of reducing friction is by A. painting B. alloying C. oiling D. braising 17. A block of wood weighs 4N lies on a horizontal table. Calculate the frictional force between the table and the wood if the coefficient of the static friction is 0.5. A. 1.0N B. 0.2N C. 2.0N D. 0.4N 18. A body of mass 2kg was moved uniformly by a force of 15N, if acceleration due to gravity is 15m/s, determine the coefficient of dynamic friction. A. 0.75 B. 1.5 C. 0.5 D. 1.75 19. The symbol for Beryllium is A. B. B. Be C. Bl D. Bm 20. K is the symbol of A. Calcium B. Chromium C. Potassium D. Krypton
Answers
Answer:
c. iron
Explanation:
iron's atomic number is 26
Select the correct answer. Which statement about cellulose is true? A. It’s a synthetic polymer. B. It’s a raw material used to make plastic. C. It’s produced using oil and gas. D. It’s used to make paper products.
Answers
Answer:
It's used to make paper.
Explanation:
Plato answer.
according to the video raw to ready: bombardier, what was added to sand by the phoenician sailors to accidently produce glass
Answers
Sand was accidentally turned into glass by the Phoenician seafarers by the use of natron.
What is the glass's chemistry?SiO2 is the primary component of flat glass (silica sand). This has a high melting point of around 1700 degrees Celsius, and in this form it resembles syrup on a very cold day.
What makes glass useful in chemistry?Glass composed of borosilicate is a leader in general analytical applications due to its straightforward composition, excellent chemical resistance, and strong thermal shock capabilities. The advantages of borosilicate glass are all present in 96% silica glass, which also has outstanding thermal shock resistance and high service temperature characteristics.
To know more about Glass visit:
https://brainly.com/question/28801236
#SPJ4
be sure to answer all parts. in the electrolysis of a molten mixture of rbf and cacl2, identify the product that forms at the negative electrode and at the positive electrode. negative electrode: rb f2 ca cl2 positive electrode: rb f2 ca cl2
Answers
To answer your question, in the electrolysis of a molten mixture of RbF and CaCl2, the product that forms at the negative electrode is Rb metal and F2 gas.
To answer your question, in the electrolysis of a molten mixture of RbF and CaCl2, the product that forms at the negative electrode is Rb metal and F2 gas. This is because the negative electrode, also known as the cathode, attracts positively charged ions, which in this case is Rb+. The Rb+ ions are reduced by gaining electrons from the cathode and form Rb metal. At the same time, the F- ions in the molten mixture are also attracted to the cathode, and they gain electrons to form F2 gas.
On the other hand, the product that forms at the positive electrode, also known as the anode, is Cl2 gas and Ca metal. This is because the positive electrode attracts negatively charged ions, which in this case is Cl-. The Cl- ions are oxidized by losing electrons at the anode to form Cl2 gas. At the same time, the Ca2+ ions in the molten mixture are also attracted to the anode, and they lose electrons to form Ca metal.
It is important to note that in electrolysis, the cathode is the electrode where reduction occurs, while the anode is the electrode where oxidation occurs. Electrodes are conductive materials that allow the flow of electricity and are used in electrolysis to transfer electrons between the solution and the power source.
To know more about electrolysis visit: https://brainly.com/question/12994141
#SPJ11
what is van der wall force? who discovered it and in what is it applied? how is it applied?
Answers
Answer:
Van de Waal forces are intermolecular forces (London Dispersion forces, Dipole-Dipole forces, and Hydrogen Bonds). It was discovered by a Dutch scientist called Johannes Diderik van der Waals. Intermolecular forces are the attraction and strength of bonds between molecules, with London Dispersion Forces being the weakest and Hydrogen Bonds being the strongest.
Explanation:
12.
Carboxylic acids react with alkalis to produce salt and water.
+
+
CH, COOH
NaOH
Ethanoic acid Sodium hydroxide
NaCH COO
Sodium ethanoate
но
Water
a. What mass of water would be produced if 1.5 g of ethanoic acid was reacted completely
with sodium hydroxide?
Show your working clearly.
3
Answers
Mass of water : 0.45 g
Further explanationGiven
1.5 g ethanoic acid
Required
Mass of water
Solution
Reaction
CH₃COOH + NaOH ⇒H₂O + CH₃COONa
mol CH₃COOH(MW=60 g/mol) :
= mass : MW
= 1.5 : 60
= 0.025
mol H₂O from the equation :
= 0.025(mol ratio CH₃COOH : H₂O= 1 : 1)
Mass of water :
= mol x MW water
= 0.025 x 18 g/mol
= 0.45 g
5. in this experiment, 1.0 ml of saturated sodium chloride is used to rinse the hickman head after the initial distillation. why is saturated sodium chloride, rather than pure water, used for this procedure and the subsequent washing of the organic layer?
Answers
Saturated sodium chloride is used in this procedure because it helps to separate the organic layer from the aqueous layer.
What is organic layer?
Organic layer is a type of soil layer that includes organic matter such as leaves, wood, roots, and other plant and animal remains. This type of soil is important for agricultural production and is also beneficial to the environment. Organic layer is also known as top soil or humus. It is the most fertile and nutrient rich soil layer, due to the presence of the decomposing organic matter. Organic layer helps to regulate soil temperature and moisture, improve soil structure and aeration, and provide essential nutrients for plant growth. It also helps to reduce erosion, improve water-holding capacity, and keep soil particles in place. Organic layer also helps to increase the biodiversity of the soil, as it provides a habitat for beneficial microorganisms and other organisms. Organic layer is an important part of any healthy soil system.
The sodium chloride solution has a higher density than water and helps to ensure that the two layers remain distinct. Additionally, the sodium chloride helps to break the surface tension between the two layers, making it easier to separate them.
To learn more about organic layer
https://brainly.com/question/14356327
#SPJ4
which is the correct formula for the compound formed between beryllium and nitrogen?
Answers
Answer:
Be3N2
Explanation:
u cross multiply with their subscripts
how many gallons of a % antifreeze solution must be mixed with gallons of % antifreeze to get a mixture that is % antifreeze? use the six-step method.
Answers
There are 490 gallons of a 70 % antifreeze solution must be mixed with gallons of % antifreeze to get a mixture that is 30 % antifreeze.
An equation can be created using the relationship between each antifreeze proportion.
The required amount of 90% antifreeze is 490 gallons.
The query is unfinished. I will thus address the query using the following information.
Antifreeze available = 70 gallons at 10%
Gallons of 90% antifreeze equal x.
Total = x + 70 to obtain an 80% solution after combining the solution.
The formula is therefore written as follows:
(x+70) x 80% = 70x 10% + x 90%
assemble similar terms
0.9x +0.8x = 56-7
0.1 x= 49
Divide by 0.1 and get 490.
Your question is incomplete but most probably your full question was
How many gallons of a 70% antifreeze solution must be mixed with gallons of 90% antifreeze to get a mixture that is 30% antifreeze? Use the six-step method.
Read more about equations at:
brainly.com/question/2264804
#SPJ4
What is the composition, in weight percent, of an alloy that consists of 94. 1 at% ag and 5. 9 at% cu? the atomic weights for ag and cu are 107. 87 g/mol and 63. 55 g/mol, respectively.
Answers
The composition, in weight percent, of the alloy is therefore 94.6% Ag and 5.4% Cu.
To calculate the composition in weight percent of an alloy that consists of 94.1 at% Ag and 5.9 at% Cu, we need to follow the steps below:
1: Calculate the molar fraction of Ag
Molar fraction of Ag = % of Ag in the alloy / atomic weight of Ag
Molar fraction of Ag = 94.1 / 107.87 = 0.8715
2: Calculate the molar fraction of Cu
Molar fraction of Cu = % of Cu in the alloy / atomic weight of Cu
Molar fraction of Cu = 5.9 / 63.55 = 0.0929
3: Calculate the total molar mass of the alloy by using the molar fractions
Total molar mass of the alloy = (molar fraction of Ag × atomic weight of Ag) + (molar fraction of Cu × atomic weight of Cu)
Total molar mass of the alloy = (0.8715 × 107.87) + (0.0929 × 63.55) = 99.49 g/mol
4: Calculate the weight fraction of each element
Weight fraction of Ag = (molar fraction of Ag × atomic weight of Ag) / total molar mass of the alloy
Weight fraction of Ag = (0.8715 × 107.87) / 99.49 = 0.946
Weight fraction of Cu = (molar fraction of Cu × atomic weight of Cu) / total molar mass of the alloy
Weight fraction of Cu = (0.0929 × 63.55) / 99.49 = 0.054
Learn more about atomic fraction at:
https://brainly.com/question/18523093
#SPJ11
what is the ph of a solution where 50.0 ml of 0.050 m nh3 (kb = 1.8 * 10-5) is mixed with 12.0 ml of 0.10 m hydrobromic acid (hbr)?
Answers
The pH of the solution where 50.0 mL of 0.050 M NH3 (Kb = 1.8 * 10-5) is mixed with 12.0 mL of 0.10 M hydrobromic acid (HBr) is 5.57.
The pH of a solution where 50.0 ml of 0.050 M NH3 (Kb = 1.8 * 10-5) is mixed with 12.0 ml of 0.10 M hydrobromic acid (HBr) can be calculated as follows:
Step 1: Write the balanced chemical equationNH3(aq) + HBr(aq) → NH4Br(aq)Step 2: Find moles of NH3 and HBrMoles of NH3 = (50.0 mL)(0.050 mol/L) = 0.0025 molMoles of HBr = (12.0 mL)(0.10 mol/L) = 0.0012 mol
Step 3: Determine which of the two reagents will run out firstNH3(aq) is a weak base and HBr(aq) is a strong acid, so they will react to form NH4+ and Br- ions. But HBr(aq) will completely dissociate in water while NH3(aq) will undergo a partial ionization. Thus, HBr will be the limiting reactant and all of the 0.0012 mol of HBr will react with 0.0012 mol of NH3 to produce NH4Br.
Step 4: Calculate moles of remaining NH3Moles of NH3 left = 0.0025 mol - 0.0012 mol = 0.0013 mol
Step 5: Calculate concentration of NH4+ ionConcentration of NH4+ ion, [NH4+] = moles of NH4+ ion/volume of solutionMoles of NH4+ ion = moles of HBr used = 0.0012 molVolume of solution = 50.0 mL + 12.0 mL = 62.0 mL = 0.062 L[NH4+] = 0.0012 mol/0.062 L = 0.019 mol/L
Step 6: Write the equilibrium equation and expression for NH4+ ionNH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq)Kb = [H3O+][NH3]/[NH4+]
Since Kb is given, we can find the Kb for NH4+ ion as follows:Kb * Kw/Ka = [H3O+][NH3]/[NH4+]1.8 * 10^-5 * 1.0 * 10^-14/5.6 * 10^-10 = [H3O+][0.0013]/[0.019][H3O+] = 2.7 * 10^-6pH = -log[H3O+]pH = -log(2.7 * 10^-6)pH = 5.57.
The pH of the solution where 50.0 mL of 0.050 M NH3 (Kb = 1.8 * 10-5) is mixed with 12.0 mL of 0.10 M hydrobromic acid (HBr) is 5.57.
To learn more about solution visit;
https://brainly.com/question/1616939
#SPJ11
Which statement best describes the chart? Heat was removed from the activation energy to create the product. Heat was added to create the product. The products have less energy than the reactants. The products have the same energy as the reactants.
Answers
Answer:
A. Heat … Get the answers you need, now! ... A. Heat was removed from the activation energy to create the product. ... C. The products have less energy than the reactants. D. The products have the same energy as the reactants.
Explanation:
Answer:
its B. Heat was added to create the product.
Explanation:
just took the test on edg2020 :)
which ion (cation or anion) remained the same between sodium sulfate and sodium chloride?
Answers
Answer:
Sodium (Na) is a cation, which means it has a positive charge.
Sodium forms ionic compounds with different anions, such as sulfate (SO4 2-) and chloride (Cl-).
In sodium sulfate (Na2SO4), sodium is still a cation with a charge of +1, while sulfate is an anion with a charge of -2.
In sodium chloride (NaCl), sodium is still a cation with a charge of +1, while chloride is an anion with a charge of -1.
Therefore, the cation that remained the same between sodium sulfate and sodium chloride is sodium (Na+).
Explanation:
Sodium (Na) is a cation, which means it has a positive charge.
Sodium forms ionic compounds with different anions, such as sulfate (SO4 2-) and chloride (Cl-).
In sodium sulfate (Na2SO4), sodium is still a cation with a charge of +1, while sulfate is an anion with a charge of -2.
In sodium chloride (NaCl), sodium is still a cation with a charge of +1, while chloride is an anion with a charge of -1.
Therefore, the cation that remained the same between sodium sulfate and sodium chloride is sodium (Na+). In chemistry, there are two types of ions: cations and anions.
Cations are ions that have a positive charge because they have lost one or more electrons. Anions are ions that have a negative charge because they have gained one or more electrons.
Sodium (Na) is a cation with a charge of +1, meaning it has lost one electron. In both sodium sulfate (Na2SO4) and sodium chloride (NaCl), sodium is still a cation with a charge of +1.
Therefore, the cation that remained the same between sodium sulfate and sodium chloride is sodium (Na+).
The cation (Na⁺) stayed the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed. The cation (Na+) remained the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed.
The ion that remained the same between sodium sulfate and sodium chloride is cation. An ion is an atom that has lost or gained one or more electrons. A positive ion is known as a cation since it has lost one or more electrons, whereas a negative ion is known as an anion since it has gained one or more electrons. The ions are crucial for the chemical reactions to occur and salt formation.
In sodium sulfate (Na2SO4), the sodium (Na) atom gives away two electrons to create Na+. In this example, the Na+ ion is formed, which is a cation. In sodium chloride (NaCl), the sodium (Na) atom gives away one electron to create Na+. In this example, the Na+ ion is also formed, which is a cation.
The cation (Na+) stayed the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed. As a result, the cation (Na+) remained the same in both cases, whereas the anions (SO₄²⁻ and Cl⁻) differed.
To learn more about cation check the link below-
https://brainly.com/question/14309645
#SPJ11
estion 2
Where are the non-metals on the periodic table?
Answers
Answer: nonmetals are located on the upper right
Explanation:
Hurry Answer for a Brainlist

Answers
Answer:
The most accurate and percise player was Josh
molecules move in random directions when heated in a heat engine, and because of the lack of uniformity in direction of molecular movement, true or false
Answers
The statement "molecules move in random directions when heated in a heat engine, and because of the lack of uniformity in the direction of molecular movement" is true.
When a heat engine is heated, molecules absorb heat energy and their kinetic energy increases. The kinetic energy of molecules causes them to move around. However, this movement is not uniform, and the molecules move in random directions.
A heat engine is a device that converts thermal energy into mechanical energy. Heat engines operate on the principle of thermodynamics.
They work by taking in thermal energy from a high-temperature reservoir, converting some of it into mechanical energy, and then releasing the remaining thermal energy to a low-temperature reservoir.The internal combustion engine in a car, the steam engine in an old locomotive, and the turbine in a power plant are all examples of heat engines. They all convert heat energy into mechanical energy to perform work.
To learn more about engine visit;
https://brainly.com/question/31140236
#SPJ11
Balance the equation using the
correct coefficient:
lithium + water produces lithium
hydroxide + hydrogen gas
[ ? ]Li + 2H2O → LiOH + H2
Enter
Answers
Answer:
\(2\; {\rm Li} + 2\; {\rm H_{2} O} \to 2\; {\rm LiOH} + 1\; {\rm H_{2}}\).
Explanation:
Assume that the coefficient of \({\rm LiOH}\) is \(1\):
\(?\; {\rm Li} + ?\; {\rm H_{2} O} \to 1\; {\rm LiOH} + ?\; {\rm H_{2}}\).
The product side of this equation would include:
\(1\) \({\rm Li\) atom.\(1\) \({\rm O}\) atom.The number of \({\rm H}\) atoms on the product side is not known since the coefficient of \({\rm H_{2}}\) is not yet found.By the conservation of atoms, the reactant side of this equation should also include:
\(1\) \({\rm Li}\) atom, and\(1\) \({\rm O}\) atom.The only reactant that includes \({\rm Li}\) atoms is \({\rm Li}\!\). Thus, the coefficient of the reactant \(\!{\rm Li}\) should be \(1\) to ensure that the reactant side of this equation includes exactly \(1\!\) \({\rm Li}\!\!\) atom.
Likewise, the only reactant that includes \({\rm O}\) atoms is \({\rm H_{2}O}\), with one \({\rm O}\!\) atom in each formula unit of \({\rm H_{2}O}\!\). There needs to be \(1\!\) \(\!{\rm O}\) atom on the reactant side of this equation. Therefore, the coefficient of the reactant \({\rm H_{2}O}\!\!\) should also be \(1\):
\(1\; {\rm Li} + 1\; {\rm H_{2} O} \to 1\; {\rm LiOH} + ?\; {\rm H_{2}}\).
The reactant side of this equation now include:
\(1\) \({\rm Li}\) atom.\(1\) \({\rm O}\) atom.\(2\) \({\rm H}\) atoms.By the conservation of atoms, the product side of this equation should also include:
\(1\) \({\rm Li}\) atom.\(1\) \({\rm O}\) atom.\(2\) \({\rm H}\) atoms.The product \(1\; {\rm LiOH}\) alone would account for \(1\; {\rm Li}\) atom, \(1\; {\rm O}\) atom, and \(1\; {\rm H}\) atom. Thus, there would be only \(1\) more \({\rm H}\) atom for the other product, \({\rm H_{2}}\). However, since there are \(2\) \({\rm H}\!\) atoms in every formula unit of \({\rm H_{2}}\!\), the coefficient of \(\!{\rm H_{2}}\) would need to be \((1/2)\):
\(1\; {\rm Li} + 1\; {\rm H_{2} O} \to 1\; {\rm LiOH} + (1/2)\; {\rm H_{2}}\).
Atoms of each element are indeed conserved in this equation.
Eliminate the fractions by multiplying all coefficients by \(2\) (least common denominator of all coefficients in this equation):
\(2\; {\rm Li} + 2\; {\rm H_{2} O} \to 2\; {\rm LiOH} + 1\; {\rm H_{2}}\).
an unknown metal has a mass of 41.7 g. when 2210 j of heat are added to the sample, the sample temperature changes by 70.6 ∘c . calculate the specific heat of the unknown metal.
Answers
The specific heat of the unknown metal is approximately 0.877 J/(g °C).
To calculate the specific heat of the unknown metal, we can use the equation:
q = mcΔT
where q is the heat energy absorbed or released, m is the mass of the metal, c is the specific heat of the metal, and ΔT is the change in temperature.
In this case, we are given that the mass of the metal is 41.7 g, the heat added is 2210 J, and the temperature change is 70.6 °C.
Plugging these values into the equation, we have:
2210 J = (41.7 g) * c * (70.6 °C)
To solve for c, we need to isolate it on one side of the equation. Dividing both sides of the equation by (41.7 g) * (70.6 °C), we have:
c = 2210 J / [(41.7 g) * (70.6 °C)]
Calculating this expression, we find:
c ≈ 0.877 J/(g °C)
To know more about specific heat visit:-
https://brainly.com/question/31608647
#SPJ11