Answers
The pressure exerted by a confined gas is the result of Option b. gas particles colliding with the walls of the container.
The pressure exerted by a confined gas is the result of gas particles colliding with the walls of the container. When a gas is confined within a container, the gas particles are in constant motion, moving in random directions with varying speeds. As these gas particles move, they collide with each other and with the walls of the container.
When a gas particle collides with the walls of the container, it exerts a force on the surface. The collective effect of numerous gas particle collisions leads to a net force being exerted on the walls of the container. This force per unit area is what we call pressure.
The more frequently and vigorously the gas particles collide with the walls, the higher the pressure of the gas. Factors that influence gas pressure include the number of gas particles present, their average speed, and the volume of the container. Therefore, Option b is correct.
The question was incomplete. find the full content below:
The pressure exerted by a confined gas is the result of
a. gas particles colliding with each other
b. gas particles colliding with the walls of the container
c. nobody knows, it just is
d. gas particles taking up space in the container.
Know more about Pressure here:
https://brainly.com/question/24719118
#SPJ8
Related Questions
2Na + 2H20 - 2NaOH + H2
→
For the reaction shown here, predict the number of moles of both products, sodium hydroxide, and hydrogen gas, when ten moles of
sodium and ten moles of water fully react
A
B)
Ten moles of sodium hydroxide and ten moles of hydrogen gas will be
produced
Ten moles of sodium hydroxide and five moles of hydrogen gas will be
produced
Five moles of sodium hydroxide and ten moles of hydrogen gas will be
produced
moride and five moles of hydrogen gas will be
Answers
Answer: B) Ten moles of sodium hydroxide and five moles of hydrogen gas will be produced
Explanation: Got it right on USATestPrep
What is the Heisenberg Uncertainty Principle?
A. We can know either the speed (momentum) or location, but not both at the same time.
B. We cannot ever know the speed (momentum) or location of the electron.
C. We can know only the speed (momentum) of the electron, but never the location.
D. We can know only the location of the electron, but never the speed (momentum).
Answers
Answer:
b option is correct
Explanation:
we cannot ever know the speed and location of the electron
2. A company makes mixtures of acetic acid and water such that the acetic acid is 15% of the total mass (weight) of the mixture. Let A be an unspecified number of grams of acetic acid, which can vary and let W be the corresponding number of grams of water in this type of mixture.
An equation that relates A and W is A = (3/17) W.
Answers
The equation that relates A and W, considering the desired 15% acetic acid concentration, is 3W = 2.55M.
The equation A = (3/17)W represents the relationship between the mass of acetic acid (A) and the mass of water (W) in the mixture. It states that the mass of acetic acid is equal to three seventeenths (3/17) of the mass of water.
Since the company wants the acetic acid to be 15% of the total mass of the mixture, we can set up another equation to represent this requirement. Let M be the total mass of the mixture. The mass of acetic acid (A) is 15% of the total mass, so we have A = 0.15M.
Now we can substitute A in terms of W from the first equation into the second equation: (3/17)W = 0.15M. We can simplify this equation by multiplying both sides by 17 to get 3W = 2.55M.
This equation allows the company to calculate the mass of water (W) required for a given mass of acetic acid (A) to maintain the desired concentration in the mixture.
For such more questions on concentration
https://brainly.com/question/26175405
#SPJ8
Which of the fields below are significantly involved in food
engineering?
A. Civil engineering, structural engineering, genetic engineering
B. Construction technology, civil engineering, structural
engineering
C. Biology, industrial engineering civil engineering
D. Genetic engineering, chemical engineering, biology
Answers
Answer:
D.
Explanation:
Genetic Engineering is a huge part in manipulating a foods property. Chemical engineering mostly the science with numbers in foods.
Biology is literally the life of the food.
Genetic engineering, chemical engineering, biology fields are significantly involved in food engineering.
Hence, Option (D) is correct answer.
What is Food Engineering ?Food engineering is the application of engineering principles which develop and design the systems for the production, storing, distributing and processing the food material.
Which fields are significantly involved in food engineering ?Food engineering is engineering field that combines microbiology, genetic engineering, chemistry engineering, biology and some other branches of science to produce food materials.
Thus from the above conclusion we can say that Genetic engineering, chemical engineering, biology fields are significantly involved in food engineering.
Hence, Option (D) is correct answer.
Learn more about the Food Engineering here: https://brainly.com/question/2868223
#SPJ2
URGENT!!! An unknown hydrate of CoCl₂ has been evaporated in a crucible. Given the following data, find the formula and name of the hydrate.
Mass of crucible: 12.090 g
Mass of hydrate before evaporation and crucible: 16.250 g
Mass of hydrate after evaporation and crucible: 12.424 g
Answers
From the given data, the name of the hydrated salt would be \(CoCl_2.83H_2O\).
Formula of hydrateThe formula of the hydrated salt can be determined using the empirical formula approach. That is, we will find the mole equivalent of the anhydrous salt and the water of hydration and then combine them into a single formula after dividing by the smallest mole.
First, we need to determine the mass of the anhydrous salt and the water of hydration.
Mass of crucible (x) = 12.090 g
Mass of hydrated salt + crucible (y) = 16.250 g
Thus, the mass of the hydrated salt can be determined by subtracting x from y.
Mass of hydrated salt = 16.250 - 12.090 = 4.16 g
Mass of hydrate + crucible after evaporating off the water (z) = 12.424 g
Mass of anhydrous salt = z - x
= 12.424 - 12.090
= 0.334 g
Mass of water = 4.16 - 0.334
= 3.826 g
Now, let's find the moles:
Molar mass of \(CoCl_2\) = 129.839 g/mol
Molar mass of water = 18.01 g/mol
Mole of \(CoCl_2\) = 0.334/129.839 = 0.00257 mol
Mole of water = 3.826/18.01 = 0.2124 mol
Dividing through by the smallest mole
\(CoCl_2\) = 0.00257 / 0.00257 = 1
water = 0.2124/ 0.00257 = 83
Thus, the formula of the hydrate would be \(CoCl_2.83H_2O\)
More on hydrate salts can be found here: https://brainly.com/question/16990374
#SPJ1
Balance the equation and indicate whether it is a synthesis reaction, a decomposition reaction, a single-displacement reaction, a double-replacement reaction, or a combustion reaction.
CH4(g)+O2(g)→H2O(g)+CO2(g)
Balanced equation:
Type of reaction:
Answers
The type of reaction between methane and oxygen to produce water and carbon dioxide is combustion reaction (option E).
What is a chemical reaction?Chemical reaction is a process, typically involving the breaking or making of interatomic bonds, in which one or more substances are changed into others.
There are different types of chemical reactions as follows;
Combination or synthesis reactionDouble-decomposition reactionDisplacement reactionCombustion reactionA combustion reaction is a reaction wherein a fuel is combined with oxygen, usually at high temperature, releasing heat.
According to this question, methane reacts with oxygen to produce water and carbondioxide. This is an example of combustion reaction.
Learn more about combustion reaction at: https://brainly.com/question/12172040
#SPJ1
A particular medication dosage is 45.0mg/kg of body weight. what is the mass in mg of the medication a child weighing 33.5lb
Answers
Answer:
Dosage of drugs is dependent on the body weight of a person. The route of administration also plays crucial role in determining the dosage of the drugs. The calculation of dosage of drugs based on the bodyweight is calculated by the multiplication of body weight and dosage.
Explanation:
The medication dosage is given as 45mg/kg. To calculate the mass of dosage a child will require of body weight 33.5lb will be:
\(\text{Dose} = \text{Body weight} \times \text{Dosage}\)
Therefore, converting the pounds to kg, it will be:
\(33.5\;\text{lb} = 15.19\;\text{ kg} \;(1\;\text{lb} = 0.4\;\text{ kg})\)
Thus, dose of medication required will be:
\(\text{Dose} = 15.19 \: \times \: 45\)
\(\text { Dose} = 683.8\: \text { mg}\)
How much did asbestos exposure decrease during the year 1982 and 1983

Answers
The asbestos exposure during the years 1982 and 1983 was 2.5 fibers per cubic centimeter and 0.8 fibers per cubic centimeter respectively. So asbestos exposure decreased by 1.7 fibers per cubic centimeter during the year 1983.
Breathlessness Persistent, dry cough Chest pain or tightness Lack of a dry, crackling sound in the lungs when you breathe in Wider and rounder fingers and toes are some of the symptoms of asbestos exposure.
The largest group of people exposed to asbestos is those working in the construction industry. Historically, asbestos was also used by pipe fitters and shipyard workers. In addition, asbestos was used by military personnel, auto mechanics, and many other occupations.
To learn more about asbestos, refer to the link:
https://brainly.com/question/8853025
#SPJ1
`In this lesson we discussed how to abbreviate electron configurations using what is called noble gas configuration. So if you were writing such a configuration for phosphorus, which noble gas would you use?
Answers
Answer:
Concept: Advanced Chemistry Techniques
Use the periodic table to locate the nearest noble gas (At the far right) P is #15 and the nearest noble gas is #10 NEWhat might have been the advantages and disadvantages of just having
experienced polar explorers at the catlin arctic survey.
Answers
While having experienced polar explorers at the Catlin Arctic Survey would have been beneficial in many ways, it would also have been important for them to work collaboratively with the rest of the team.
Advantages:
Experienced polar explorers would have had a wealth of knowledge and skills, such as how to travel over the ice, how to set up camp, and how to handle emergencies.
Experienced polar explorers would have been able to make informed decisions about the best routes to take and the most efficient ways to travel. This could have helped to save time and energy.
Disadvantages:
Experienced polar explorers may have been set in their ways and resistant to new ideas. This could have hindered the team's ability to adapt to changing circumstances and make the most of new opportunities.
Experienced polar explorers may have been overconfident and taken risks that the rest of the team was not comfortable with. This could have put everyone's safety at risk.
To learn more about the Catlin Arctic Survey, follow the link:
https://brainly.com/question/28313250
#SPJ1
What energy allows for the difference in the spacing of molecules in a liquid versus a gas?
A. Electrical energy
B. Radiant energy
C. Potential energy
D. Kinetic energy

Answers
Answer:
kinetic energy
Explanation:
yeah it's correct
It was shown that 150 J was required to raise the temperature of 20.0 g of an unknown metal from 30°C to 50°C. Using a table of specific heat
capacities, identify the unknown metal.
Answers
Answer:
0.375 J/g°C
Brass
Explanation:
To identify the unknown metal, you first need to calculate the specific heat capacity using the following equation:
Q = mcΔT
In this equation,
-----> Q = heat energy (J)
-----> m = mass (g)
-----> c = specific heat capacity (J/g°C)
-----> ΔT = change in temperature (°C)
You can find the specific heat capacity by plugging the given values into the equation and simplifying to find "c".
Q = 150 J c = ? J/g°C
m = 20.0 g ΔT = 50°C - 30°C = 20°C
Q = mcΔT <----- Given equation
150 J = (20.0 g)c(20°C) <----- Insert values
150 J = (400 g°C)c <----- Multiply 20.0 g and 20°C
0.375 J/g°C = c <----- Divide both sides by 400 g°C
The specific heat capacity of the unknown metal is 0.375 J/g°C. According to my research, there is no metal with this exact specific heat capacity. However, the closest I could find was brass (0.377 J/g°C).
Calculate the energy of a photon of radiation with a frequency of 8.5 x 10¹ Hz
Use this calculator to submit your answer in a decimal form.
Type your answer...
Answers
Answer: 0.85 hertz
Explanation: Calculator said so also 0.85 is decimal form for 85
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. In essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. Suppose a current of 0.270 A is passed through an electroplating cell with an aqueous solution of Ag_2 SO_4 in the cathode compartment for 72.0 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
Mass of the pure silver deposited on a metal object made into the cathode of the cell is calculated to be 0.0217 gm.
What is electroplating?The process of using electrodeposition to coat an object in a layer of metal is called electroplating .
As we know that, Q = I * t
=0.270 * 72
= 19.44 C
Here Q is quantity of electricity , I is current in amperes = 0.270 A (given)
t is time in seconds (72.0 sec)
As 96500 Coulomb of electricity electrolyzes 1 mole of Ag
then,19.44 C of electricity deposits,
=1/96500 * 19.44
= 0.000201 moles of Ag
Mass of Ag is = number of moles * molar mass
= 0.000201 * 108
= 0.0217 gm
Thus, mass of pure silver deposited on a metal object made into the cathode of the cell is 0.0217 gm.
To know more about electroplating, refer
https://brainly.com/question/16266707
#SPJ4
Elements in the alkaline earth metal family of the periodic table
have
electronegativity values than elements in the
halogen family of the periodic table.
Answers
Answer:Lower
Explanation:
( just had the question on ck12.)
Elements in the alkaline earth metal family of the periodic table have lower electronegativity values than elements in the halogen family of the periodic table as they have less electrons in their valence shell.
What is periodic table?Periodic table is a tabular arrangement of elements in the form of a table. In the periodic table, elements are arranged according to the modern periodic law which states that the properties of elements are a periodic function of their atomic numbers.
It is called as periodic because properties repeat after regular intervals of atomic numbers . It is a tabular arrangement consisting of seven horizontal rows called periods and eighteen vertical columns called groups.
Elements present in the same group have same number of valence electrons and hence have similar properties while elements present in the same period show gradual variation in properties due to addition of one electron for each successive element in a period.
Learn more about periodic table,here:
https://brainly.com/question/11155928
#SPJ2
How is energy related to the change of state represented by the model?
Atoms gain energy as a solid changes to a liquid.
Atoms gain energy as a solid changes to a gas.
Atoms lose energy as a solid changes to a liquid.
Atoms lose energy as a solid changes to a gas.
Answers
Answer:
Atoms gain energy as a solid changes to a liquid. If atoms energy during a change of state, they are pulled together by attractive forces and become more organized.
Answer:
its B
Explanation:
The mass % of C in methane (CH4) is?
Answers
Answer:
74.87% Carbon
Explanation:
The molecular mass of CH4 is 16.042 g/ mole.
X=(100 × 12.01) / 16.042= 74.87%
Answer:
\(\boxed {\boxed {\sf 74.87 \% \ C}}\)
Explanation:
We want to find the mass percent composition of carbon in methane: CH₄
First, we must calculate the gram formula mass, also called the molar mass. Use the values for mass found on the Periodic Table. Look for carbon and hydrogen.
C: 12.011 gH: 1.008 gThere is no subscript after C, so there is just 1 atom. There is a subscript of 4 after H, so there are 4 atoms of hydrogen. We must multiply hydrogen's mass by 4.
C: 12.011 g H₄: 1.008 g * 4= 4.032 g CH₄= 12.011 g+ 4.032 g=16.043 gCalculate the percent composition.
\(\frac {mass \ of \ part}{mass \ of \ whole} *100\)
The part is the carbon, or 12.011 grams.
The whole is the entire compound, CH₄, or 16.043 grams.
\(\frac { 12.011 \ g }{ 16.043 \ g} *100\)
\(0.748675435*100\\74.8675435\)
Let's round to the nearest hundredth. The 7 in the thousandth place tells us to round the 6 to a 7.
\(74.87 \% \ C\)
The mass percent of carbon is 74.87%
pls help i will mark brainliest Part D
Look back at your answer to part A. So far, you haven't added anything to the flask or removed
anything from it. Also, the contents of the flask have returned to the same temperature that they
were in part A. Considering the appearance of the solution in the flask, is the solution unsaturated,
saturated, or supersaturated? Explain your reasoning.

Answers
Answer:
Hello, Im Mack. Hope you're doing well. :)
Here is my Correct, custom answer for the lab Question, (I also took the same assignment and got a 100% score)
Explanation:
The heating of the sodium acetate solution made it change from super-saturated to un-saturated. Yet now that it cooled back to room temperature Im sure it is saturated. it wasn't able to disolve the excess sodium acetate left over at room temperature and had to be heated so now that it is back to room temperature I Think it will not disolve the sodium acetate left in the solution until it is heated again.
Hope this helped you out,
Please feel free to comment for further help, and I'll reply asap.
Have a great day my friend :)
The electron in Figure 6.15 is promoted even further to an orbit with
n=6 . What is its new energy?
Answers
Without the specific figure 6.15, I am unable to provide an exact answer to this question. However, the energy of an electron in an atom is determined by its principal quantum number (n).
Which represents the electron's energy level. The energy of an electron increases as its principal quantum number increases.
The energy of an electron in a particular energy level (n) can be calculated using the formula: E = -13.6 eV/n^2, where eV represents electron volts. This formula is derived from the Bohr model of the atom.
So, if an electron in a lower energy level (such as n=2) is promoted to a higher energy level (such as n=6), the energy of the electron increases accordingly. The energy of the electron in its new energy level can be calculated using the formula above.
Note that the energy levels of electrons in real atoms are more complex than the simplified Bohr model, and the energy of an electron is influenced by many factors beyond its principal quantum number.
To know more about quantum number, visit :
https://brainly.com/question/16977590
#SPJ1
True or False
Helium belongs to Noble Metals
Answers
Answer:
This answer is "True"
Rank from most ionic to least ionic
a. WO3
b. MnS
c. MnS2
d. ZnS
e. ZrS2
Answers
The order of increasing ionic property is;
WO3 < ZrS2 < MnS2< ZnS < MnS
Ionic compounds are compounds that contain an ion pair. Typically, ionic compounds are formed between metals and nonmetals.
The degree of ionic character depends on the type of metal involved and the magnitude of charge it carries.
Typically, first row transition metals form ionic compounds. The degree of ionic character depends on the row in which the metal is found and the magnitude of charge it carries.
Hence, the order of ionic character of the compounds from most ionic to least Ionic is; WO3 < ZrS2 < MnS2< ZnS < MnS.
Learn more;https://brainly.com/question/25150590
Trend of atomic number and atomic size of the elements when we move from left to right in different periods of periodic table
Answers
Answer:
The atomic size decreases with an increase in atomic number when we move from left to right.
Explanation: Hope it helps you:))))))
Have a great day.
What is chemistry
What is the bond type in CaO
Answers
Answer:
Ionic bond
CaO is an ionic bond. Two-element compounds are usually ionic when one element is a metal and the other is a non-metal. It is made up of one metal ion/cation(Ca^2+) and an non-metal ion/anion(O^2-).
What is the mass of a cube of Gold that is 2.5 in. on each side. According to the Periodic Table, the density of Gold is 19.3 g/ml. Find the mass of the cube in kg.
Answers
Answer: dunno but thanks for the points xd
Explanation:
What is the mass of 1.81 x 1023
molecules of nitrogen, N2? The molar
mass of N2 is 28.02 g/mol.
Answers
The mass of nitrogen, N₂ that contains 1.81×10²³ molecules is 8.42 g
How do I determine the mass of nitrogen, N₂?From Avogadro's hypothesis,
6.02×10²³ molecules = 1 mole of nitrogen, N₂
But
1 mole of nitrogen, N₂ = 28.02 g
Thus,
6.02×10²³ molecules = 28.02 g of nitrogen, N₂
With the above information, we can obtain the mass of nitrogen, N₂ that contains 1.81×10²³ molecules as follow:
6.02×10²³ molecules = 28.02 g of nitrogen, N₂
Therefore,
1.81×10²³ molecules = (1.81×10²³ × 28.02) / 6.02×10²³
1.81×10²³ molecules = 8.42 g of nitrogen, N₂
Thus, we can conclude that the mass of nitrogen, N₂ is 8.42 g
Learn more about mass and number of molecules:
https://brainly.com/question/28855850
#SPJ1
what’s Nacl molar mass amount
Answers
NaCl is made of Na and Cl
The molar mass of Na = 22.989 g/mol
The molar mass of Cl = 35.453 g/mol
Molar mass of NaCl = 22.989 + 35.453
= 58.44 g/mol
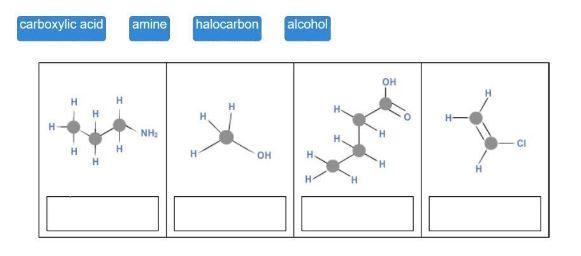
Match each hydrocarbon class to its structure.
4
carboxylic acid
H
H
HT
H
H
H
amine
NH₂
halocarbon
H
OH
alcohol
H.
H
H.
'H
OH
'H
H-
-CI
Answers
The tile's suggested answers include amine, alcohol, carboxyl group, and halocarbon.
Gasoline is it a hydrocarbon?Hydrocarbons are organic substances comprised of hydrogen and carbon, and include petroleum, methane gas, and coal. Alkanes are both a highly combustible chemical and the main source of fuel in the planet. Its uses include diesel, jet fuel, propane, petrol, and petroleum, to name a few.
What makes it a hydrocarbon?The most fundamental category of organic compounds is referred to as a hydrocarbon. As their name implies, they are exclusively made up of the elements hydrogen and carbon. Atoms surround one or more core carbon atoms in hydrocarbon molecules, which are branching or chain-like in shape.
To know more about Hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ1
The complete question is-
Drag each tile to the correct image. Match each hydrocarbon class to its structure. carboxylic acid amine halocarbon alcohol.
The helium tank has a pressure of 650 torr at 25 degree celsius what will be the pressure if the temperature is tripled?
pa help po
Answers
The helium tank has a pressure of 650 torr at 25 degree Celsius and when the temperature is tripled, the pressure will be approximately 1945.71 torr
To find the new pressure when the temperature is tripled, we can use the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature when the volume and the number of particles remain constant. The ideal gas law is given by the equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
First, we need to convert the initial temperature of 25 degrees Celsius to Kelvin. Adding 273.15 to the Celsius temperature gives us 298.15 K.
Let's assume that the volume, number of moles, and the gas constant remain constant.
If the temperature is tripled, the new temperature would be 3 times the initial temperature, which is 3 * 298.15 K = 894.45 K.
Now, we can set up a proportion to find the new pressure:
P1 / T1 = P2 / T2
Solving for P2 (the new pressure), we get:
P2 = (P1 * T2) / T1
Plugging in the values, we have:
P2 = (650 torr * 894.45 K) / 298.15 K
Calculating this expression, we find:
P2 ≈ 1945.71 torr
Therefore, when the temperature is tripled, the pressure will be approximately 1945.71 torr.
for more questions on pressure
https://brainly.com/question/24719118
#SPJ11
What was the average speed of the truck for this trip?

Answers
Answer:
31.67 mph
Explanation:
To calculate the average speed of the truck, we must first obtain the total distance travelled by the truck followed by the total time taken for the truck to cover the distance travelled.
The following data were obtained from the question include:
Total distance) = 30 + 45 + 50 + 65 = 190 miles
Total time = 1 + 2 +1 +2 = 6 hours
Average speed =.?
Average speed = Total distance / Total time
Average speed = 190 /6
Average speed = 31.67 mph
Therefore, the average speed of the truck is 31.67 mph
Is this equation balanced?
3kBr + FeCl3 -> FeBr + 3KCl
A) No, it’s not balanced. The K atoms are not balanced.
B) Yes, it’s balanced.
C) No, it’s not balanced. The Br atoms are not balanced.
D) No, it’s not balanced. The Fe atoms are not balanced.
Answers
The balanced equation should be:
3KBr + FeCl3 → FeBr3 + 3KCl
In the balanced equation, both the number of Fe and Br atoms are equal on both sides of the equation.
Answer:
The given equation is not balanced.
On the left-hand side, there are 3 atoms of potassium (3K), 3 atoms of bromine (3Br), and 1 atom of iron (Fe).
On the right-hand side, there is 1 atom of bromine (Br), 3 atoms of potassium (3K), and 1 atom of iron (Fe).
The number of atoms of each element should be equal on both sides of the equation. Therefore, to balance the equation, we need to adjust the coefficients of the molecules.
The balanced equation is:
3kBr + FeCl3 -> FeBr3 + 3KCl
The balanced equation has 3 atoms of potassium (3K), 3 atoms of bromine (3Br), 1 atom of iron (Fe), and 3 atoms of chlorine (3Cl) on both sides of the equation.