The gas pressure inside a container decreases when which of the following happens? the number of molecules is increased and the temperature is increased the number of gas molecules is decreased the temperature is increased the number of gas molecules is increased
Answers
Related Questions
If the wavelengths or light absorbed by hydrogen and helium are shifted toward the red end of the
spectrum, are those wavelengths becoming shorter or longer? Explain
Answers
Answer:
Wavelengths towards the red end of the spectrum has a longer wavelength
Explanation:
As seen by this diagram, we can tell that red has the longest wavelength than any other colour. Red has a lower frequency and so carries less energy.
Hope this helps!
Complete the ion symbol for the
atom below.

Answers
Answer:Helium
Explanation:
Helium can hold two electrons on the outer shell. as for the inner part it mostly looks like helium.
Where are the transition metals on the periodic table?
A. In the first column
B. In the second column
c. In two rows, separated from the rest of the table
D. In columns 3 - 12 in the center of the table
SUBMI
Answers
Answer:
D.
Explanation:
In columns 3 - 12 in the center of the table
How many liters of carbon dioxide can be “scrubbed”, or removed, with 3.45 Liters of 0.10 M of lithium hydroxide solution?
Answers
Answer:
The correct answer is - 3.864 L of CO2.
Explanation:
1. How many molecules of H,O are in 4.32 moles?
Answers
Answer:
dont know
Explanation:
answer pls ill give brainly
How is energy from the sun used as food energy by organisms in an ecosystem?
Energy from the sun is absorbed as heat by consumers.
Energy from the sun is absorbed by fungi, and it is then passed on to consumers.
Energy from the sun is converted into food energy by producers, which is then passed on to consumers.
Energy from the sun is converted into food by consumers, which is then passed on to producers.
Answers
Answer:
its absorbed by heat from consumers
What effects do catalysts have on chemical reactions?
1.Catalysts slow down chemical reactions
2.Catalysts reverse chemical reactions
3.Catalysts speed up chemical reactions
4.all of the above
Answers
Answer:
3
Explanation:
At a fixed temperature and pressure, the volume occupied by a gas is _____ proportional to the number of moles of gas present. For ideal gases under these conditions, equal _____ of gas contain equal numbers of particles or moles.
Answers
Answer:
that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules." For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
find the area given rectangle.
The length and and breadth of a rectangle are 3abc³ and 4abc units. The area of rectangle is __ sq.unit .
Answers
Answer:
The perimeter of a Rectangle P= 2(l + b)
Area of a Rectangle A = l × b
I would need some help on this I would really appreciate if you could help out with that one.
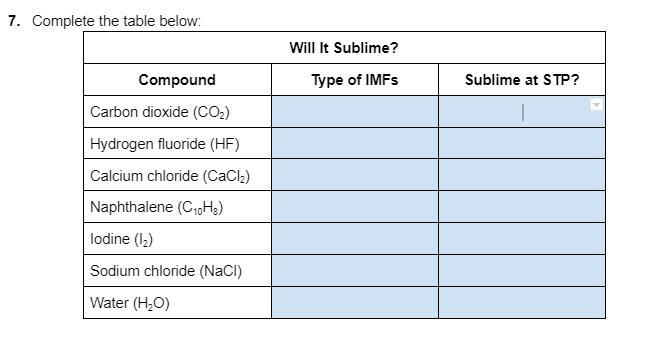
Answers
The types of intermolecular forces (IMFs) present in Carbon dioxide are London dispersion forces and dipole-dipole forces. At standard temperature and pressure (STP), which is defined as 0°C and 1 atm, carbon dioxide will sublime.
What are different types of intermolecular forces?There are several types of intermolecular forces, including London dispersion forces, dipole-dipole interactions, and hydrogen bonding.
(1) Carbon dioxide :
Type of IMFs : London dispersion forces.
Sublime at STP : Yes
(2) Hydrogen fluoride :
Type of IMFs : Dipole- Dipole
Sublime at STP : No
(3) Calcium chloride :
Type of IMFs : Ionic
Sublime at STP : No
(4) Naphthalene :
Type of IMFs : London dispersion forces
Sublime at STP : Yes
(5) Iodine :
Type of IMFs : Dipole induced dipole
Sublime at STP : Yes
(6) Sodium chloride :
Type of IMFs : Ionic
Sublime at STP : No
(6) Water :
Type of IMFs : Hydrogen Bonding
Sublime at STP : No
Learn more about forces here:
https://brainly.com/question/9007693
#SPJ1
what's the particle that allowed elements to be arranged in the order of their atomic number
Answers
Answer:
protons
Atomic number and protons
After the discovery of protons , scientists realised that the atomic number of an element is the same as the number of protons in its nucleus . In the modern periodic table, the elements are arranged according to their atomic number - not their relative atomic mass .
Predict the polarity of 6 real molecules (O2, HF, H2O, NH3, CF4, CH3F). First, draw the
molecules and any bond dipoles. Then draw any molecular dipoles. Explain your
reasoning before you check your predictions with the simulation.
Answers
Carbon dioxide,Ammonia,Oxygen,Water,Hydrogen fluoride,Nitrogen are the polarity of 6 real molecules
What are the molecular polarity?Polar molecules are those that possess regions of positive and negative charge. Water is an example of a polar material. The type of bonds it has, when coupled with its shape, gives one end of the molecule a slight positive charge (the hydrogen end) and the other a slight negative charge (the oxygen end).Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge.Whether a molecule is polar or nonpolar is a matter of its geometry. If one end of the molecule has a positive charge while the other end has a negative charge, the molecule is polar. If a charge is evenly distributed around a central atom, the molecule is nonpolar.To learn more about molecular polarity refers to:
brainly.com/question/17118815
#SPJ1
compared with a 0.01 sugar solution, a 0.01 nacl solution has
Answers
A 0.01 sugar solution and a 0.01 NaCl solution have different properties and characteristics due to the different nature of the solutes present in the solutions.
A sugar solution refers to a solution in which a sugar, such as glucose or sucrose, is dissolved in water. The concentration of the sugar in the solution is measured in molarity, which is defined as the number of moles of solute per liter of solution. A NaCl solution refers to a solution in which sodium chloride (NaCl) is dissolved in water. The concentration of the NaCl in the solution is also measured in molarity.
The properties of a solution depend on the nature of the solute and the concentration of the solution. In general, as the concentration of a solution increases, the solute particles become more dispersed and the solution becomes more dilute. This can lead to changes in the physical and chemical properties of the solution, such as changes in color, odor, taste, and viscosity.
Learn more about sugar solution visit: brainly.com/question/30061259
#SPJ4
PLEASE HELP WITH CHEM CLASS!!!!!
Please please please !! Explain depth. Please !!!!

Answers
Answer:
The answers to your questions are given below
Explanation:
1. KClO3 => Potassium trioxochlorate (V)
Thermal decomposition of KClO3.
When we heat potassium trioxochlorate (V), KClO3, it will slip into two producing potassium chloride, KCl and oxygen gas, O2 as shown below:
2KClO3(s) —> 2KCl(s) + 3O2(g)
Note: the above reaction will occur faster and at a lower temperature in the presence of manganese (iv) oxide as catalyst.
2a. Determination of the number protons and neutrons in Nitrogen–14.
Nitrogen–14 has the following:
Mass number = 14
Atomic number = 7
Proton =?
Neutron =?
Atomic number of an atom is simply the proton number. Therefore,
Proton = Atomic number
Atomic number = 7
Proton = Atomic number = 7
Proton = 7
Mass number = Proton + Neutron
Mass number = 14
Proton = 7
Neutron =?
14 = 7 + Neutron
Collect like terms
Neutron = 14 – 7
Neutron = 7
Therefore, Nitrogen–14 has 7 protons and 7 neutrons.
2b. Determination of the number protons and neutrons in Nitrogen–15.
Nitrogen–15 has the following:
Mass number = 15
Atomic number = 7
Proton =?
Neutron =?
Atomic number of an atom is simply the proton number. Therefore,
Proton = Atomic number
Atomic number = 7
Proton = Atomic number = 7
Proton = 7
Mass number = Proton + Neutron
Mass number = 15
Proton = 7
Neutron =?
15 = 7 + Neutron
Collect like terms
Neutron = 15 – 7
Neutron = 8
Therefore, Nitrogen–15 has 7 protons and 8 neutrons.
Plz plz help
Which model shows 5 electrons in the outer shell of the atom?
A.2
B.3
C.4
D.1

Answers
Answer:
D
Explanation:
there are 5 electrons in the outer shell of the first picture
According to electronic configuration, model 1 shows 5 electrons in the outer shell of the atom.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/29757010
#SPJ2
A wagon is pushed in a straight line. How would an increase in friction on the wagon wheels affect the wagon ?
Answers
Answer:
A wagon is pushed in a straight line. How would an increase in friction on the wagon wheels affect the wagon? A It would cause the wagon to move faster.
Explanation:
Hoped this helped you guys!
what gas reacts with fossil fuels when burned for energy? A. oxygen b. natural gas
c. hydrocarbon d. carbon dioxide
Answers
When fossil fuels are mined and used for energy, carbon dioxide gas combines with them to form an essential heat-trapping gas known as a greenhouse gas.
The correct answer is D
Are people harmed by carbon dioxide?Many health impacts from CO2 exposure may occur. These symptoms could include a coma, hypoxia, convulsions, sweating, disorientation, agitation, a tingling or pins-and-needles sensation, difficulty breathing, fatigue, increased heart rate, and high blood pressure.
What is the principal function of carbon dioxide?As a significant greenhouse gas, carbon dioxide aids in keeping heat in the atmosphere. Our earth would be icy and inhospitable without it. But as CO2 levels grow in the atmosphere, average global temperatures rise as well, affecting other facets of Earth's climate.
To know more about carbon dioxide visit:
https://brainly.com/question/3049557
#SPJ4
0. A radioactive isotope has a half-life of 273 days. How much of a sample of 100 grams of the isotope would remain after 732 days?
Answers
The amount of a sample of 100 grams of a radioactive isotope that would remain after 732 days would be 14.0625 grams.
Given, the Half-life of the radioactive isotope = 273 days.Time elapsed = 732 days.Initial quantity or sample = 100 grams. Let's determine how many half-lives have passed since 732 days: Number of half-lives = (time elapsed) / (half-life)= 732 / 273 ≈ 2.683
Half-life #1: After the first half-life of 273 days, the sample will be halved. Therefore, after 273 days, the quantity remaining will be 1/2 * 100g = 50g
Half-life #2: After the second half-life of 273 days, the sample will be halved again. Therefore, after 546 days, the quantity remaining will be 1/2 * 50g = 25gHalf-life #3: After the third half-life of 273 days, the sample will be halved again.
Therefore, after 819 days, the quantity remaining will be 1/2 * 25g = 12.5gHowever, the time elapsed from 819 days to 732 days is 87 days. This time interval is less than the half-life. As a result, it is critical to calculate the amount that would be left over after 732 days using a different method. Let us consider the remaining amount from 819 days (12.5g) as the new initial quantity for the remaining 87 days. The half-life of the radioactive isotope is 273 days.
Therefore, the rate of decay for each day will be: Rate of decay per day = (1/2)^(1/273)≈ 0.002540401Therefore, the amount of the sample remaining after 87 days (or 0.3195 half-lives) can be calculated using the following formula: Q = Q0(0.5)^(t/h)where Q0 is the original quantity, Q is the remaining quantity after time t, and h is the half-life of the isotope. Q = 12.5g × (0.5)^(0.3195)Q ≈ 6.5625g
Therefore, the total amount of the sample remaining after 732 days can be found by adding up the amounts of the sample remaining from each half-life: Total remaining = 50g + 25g + 6.5625gTotal remaining ≈ 81.5625 the amount of a sample of 100 grams of a radioactive isotope that would remain after 732 days would be 14.0625 grams.
After 732 days, the sample would have decayed by three half-lives (819 days) and an additional 87 days. As a result, 81.5625g of the sample will remain after 732 days. Therefore, 100g - 81.5625g = 18.4375g of the sample would have decayed in 732 days.
To know more about isotopes, visit:
https://brainly.com/question/14220416
#SPJ11
How many atoms of titanium are there in 0.820 mole of each of the following? 1st attempt Part 1 (1point) ilmenite, FeTiO 3
Ti atoms Part 2 titanium(IV) chloride Ti atoms Part 1 ilmenite, FeTiO 3
Ti atoms Part 2 titanium(IV) chloride Ti atoms
Answers
To determine the number of atoms of titanium in 0.820 mole of each compound, we need to use Avogadro's number, which is 6.022 x 10²³ atoms/mol.
1. Ilmenite, FeTiO3:
In 1 mole of FeTiO3, there is 1 mole of titanium atoms.Therefore, in 0.820 mole of FeTiO3, there are 0.820 moles of titanium atoms.The number of titanium atoms in 0.820 mole of ilmenite is 0.820 x 6.022 x 10²³ atoms.2. Titanium(IV) chloride, TiCl4:
In 1 mole of TiCl4, there is 1 mole of titanium atoms.Therefore, in 0.820 mole of TiCl4, there are 0.820 moles of titanium atoms.The number of titanium atoms in 0.820 mole of titanium(IV) chloride is 0.820 x 6.022 x 10²³ atoms.Thus, the number of titanium atoms in 0.820 mole of ilmenite is 4.917 x 10²³ atoms, and the number of titanium atoms in 0.820 mole of titanium(IV) chloride is 4.917 x 10²³ atoms.
To learn more about Avogadro's number, Visit:
https://brainly.com/question/859564
#SPJ11
c) Can two electrons have same set of all four quantum numbers? Justify.
Answers
It is not possible for two electrons to have the same set of all four quantum numbers in an atom, as it would violate the Pauli exclusion principle.
According to the Pauli exclusion principle, no two electrons in an atom can have the same set of all four quantum numbers. The four quantum numbers used to describe an electron's state are the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s).
The principal quantum number (n) determines the energy level of an electron and can have integer values starting from 1. The azimuthal quantum number (l) determines the shape of the electron's orbital and can have values from 0 to (n-1). The magnetic quantum number (m) determines the orientation of the orbital and can range from -l to +l. The spin quantum number (s) describes the spin of the electron and can have two possible values, +1/2 or -1/2.
Since each electron in an atom must occupy a unique set of quantum numbers, they must differ in at least one of the four quantum numbers. This ensures that no two electrons have the exact same quantum state.
For such more questions on atom
https://brainly.com/question/6258301
#SPJ8
The fact that no chemical bonding occurs between the components of a mixture is the chief difference between mixtures and compounds. The fact that no chemical bonding occurs between the components of a mixture is the chief difference between mixtures and compounds.
a. True
b. False
Answers
The statement is (b) false. The chief difference between mixtures and compounds is not solely based on whether chemical bonding occurs between their components.
A mixture is a combination of two or more substances that are physically intermingled but not chemically bonded. In a mixture, the substances retain their individual properties, and they can be separated by physical means.
Examples of mixtures include air (a combination of gases), saltwater (a combination of salt and water), and a salad (a combination of various ingredients).
On the other hand, a compound is a substance composed of two or more elements that are chemically bonded together in a fixed ratio. Compounds have unique properties that are different from their constituent elements, and they can only be separated into their elements through chemical reactions.
Examples of compounds include water (H2O), table salt (NaCl), and carbon dioxide (CO2).
Therefore, the chief difference between mixtures and compounds lies in their composition and the nature of the interactions between their components.
In mixtures, no chemical bonding occurs between the components, whereas compounds are formed by chemical bonding between elements.
It's important to note that while the absence of chemical bonding is a significant difference, it is not the sole defining characteristic that distinguishes mixtures from compounds.
Other factors such as composition, properties, and separability are also essential in classifying substances as mixtures or compounds.
To know more about mixture visit:
https://brainly.com/question/24647756
#SPJ11
Question 2
1 points
Alkynes unlike their cousins alkenes, have two types of reactivity namely addition reaction to the triple bond and substitution reaction through acetylenic anion
Answers
Alkynes react in two ways: addition to the triple bond and substitution through the acetylenic anion.
1. Addition reaction to the triple bond: Alkynes can undergo addition reactions where atoms or groups of atoms add to the triple bond, forming a single bond. For example, hydrogenation of alkynes with hydrogen gas (H2) and a metal catalyst can produce an alkane.
2. Substitution reaction through the acetylenic anion: Alkynes can also undergo substitution reactions, where a functional group replaces another atom or group of atoms in the molecule. In the case of alkynes, the acetylenic anion (-C≡C:^-) can be substituted with other groups. For example, the acetylenic anion can react with an alkyl halide to form an alkene.
Overall, alkynes exhibit both addition and substitution reactions, providing them with diverse reactivity compared to alkenes.
Learn more about Alkynes here:
https://brainly.com/question/24270289
#SPJ11
Patience, a popular card game in Mendeleev's time, is also known as Go Fish.
A. True
B. False
Answers
Answer:
B. False
Explanation:
step by step explanation
Answer:
False
Explanation:
the amount of fluorine in a metal fluoride is 14.96%. 2 Chromium are connected to the metal when metal chromate is formed. What is the relative atomic mass of the metal
Answers
Answer:
The relative atomic mass of the metal is 207.2 u
Explanation:
Metal chromate
Given that;
1) The mass of fluorine is 14.96% of the metal fluoride
2) 2 Chromium are connected to the metal when the metal chromate, CrO²⁻, is formed
We have;
Number of ions available in the metal = Cr₂O₇²⁻ = +2 ionic
Molar mass of fluorine = 18.998 g/mole
Ionization of fluorine = -1
Number of moles of fluorine required per metal +2 ion= 2 moles
3) Number of moles of fluorine per mole of compound of the metal fluoride = 2 × moles
Mass of fluorine per mole of compound = 2 × 18.998 = 37.996 grams
Percentage by mass of fluorine = 14.96%
Fluoride
Let the mass of the compound = X
Therefore;
14.96% of X = 37.996 grams
X = 37.996/(0.1496) = 253.984 grams
Therefore the mass of the metal in the compound = 253.984 - 37.996 = Molar mass 215.99 grams
Given that the metal forms a chromate with 2 chromium atoms and a mass of 215.99 grams, the likely candidate is lead, Pb with a molar mass of 207.2 grams and a chromate of Pb(CrO₄)₂.
The fluoride, lead fluoride, F₂
The relative atomic mass of lead is 207.2 u
How would you name this?
*Grade 12 organic chemistry*

Answers
According to IUPAC nomenclature, the longest chain of this compound contains 7 carbons. Hence, the name of this compound is 4 -propyl-5-ethyl-5-phenyl heptane.
What is IUPAC nomenclature?IUPAC rules are used to name the organic compounds. As per this rule, the compound is named based on the chain containing highest number of carbon atoms. The substituents are named with their prefix or suffix.
For the given compound, the longest chain contains 7 carbon atoms. Hence, the chain is named as heptane. The substituents are numbered in such a way that, they have the least possible number.
The 4th carbon contains a propyl group and a phenyl group and ethyl groups are attached on the 5th position in the longest chain. Therefore, the name of the compound is 4 -propyl-5-ethyl-5-phenyl heptane.
Find more on IUPAC rules:
https://brainly.com/question/27915542
#SPJ1
How do we solve this? I got B but answer key says A

Answers
The concentration of NH3 at equillibirium is 0.00010M. Option A.
Ammonium nitrate is formed when nitric acid reacts with ammonia. It is a white crystalline solid consisting of ammonium ions and nitrate ions. Soluble in water, but does not form hydrates. Ammonia is directly neutralized with sulfuric acid to produce ammonium sulfate.
The neutralization evaporator and crystallizer are connected so that the heat released during neutralization is used to evaporate the water in the ammonium sulfate slurry. These units operate under partial vacuum. Nitric acid pH neutralization is common and any inorganic base such as sodium hydroxide or lime can be used. Ammonia gas reacts with hydrogen chloride gas to form ammonium chloride.
Learn more about Nitrous acid here:-https://brainly.com/question/25752475
#SPJ9
9! Direction: Create a K-W-H-L Chart (as seen below) and fill it to assess your prior knowledge and understanding of the topic. W H K L What do I know? What do I want to find out? How can I find out what I want to learn? What did 1 learn? Skills I expect to use:
sana masagot nyo
Answers
Answer:
what i don't understand
e. Which group on the periodic table has the largest number of elements? Hint the answer is not metals or nonmetals it’s more specific
Answers
Group on the periodic table has the largest number of elements is group 3
The periodic table is a tabular array of the chemical elements organized by atomic number from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number and the atomic number of an element is the number of protons in the nucleus of an atom of that element
Group 3 which comes under transition metals is the longest group consisting 32 elements and it contains lanthanide means 57 to 71 and actinide series means 89 to 103 and this comes under f block family
Know more about periodic table
https://brainly.com/question/28177183
#SPJ1
A quantity of Xe occupies 321 mL at 300 oC and 2.09 atm. What will be the temperature if the volume is increased to 553 mL at 305 torr?259 K586 K134 K189.5 K306 K
Answers
The temperature if the volume is increased to 553 mL at 305 torr will be 189.5 K.
To solve this problem, we can use the combined gas law equation, which relates the initial and final conditions of pressure, volume, and temperature. The equation is as follows:
(P1V1/T1) = (P2V2/T2)
Where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
We are given that the initial conditions are:
P1 = 2.09 atm
V1 = 321 mL
T1 = 300 K
We are also given that the final conditions are:
P2 = 305 torr (which we need to convert to atm)
V2 = 553 mL
To convert torr to atm, we divide by 760 torr/atm:
305 torr ÷ 760 torr/atm = 0.4013 atm
Substituting the values into the equation, we get:
(2.09 atm)(321 mL)/(300 K) = (0.4013 atm)(553 mL)/(T2)
Simplifying the equation, we get:
T2 = (0.4013 atm)(553 mL)(300 K)/(2.09 atm)(321 mL) = 189.5 K
Therefore, the final temperature is 189.5 K.
The question could be rephrased as:
A quantity of Xe occupies 321 mL at 300 oC and 2.09 atm. What will be the temperature if the volume is increased to 553 mL at 305 torr?
1. 259 K
2. 586 K
3. 134 K
4. 189.5 K
5. 306 K
Learn more about gas law at: https://brainly.com/question/27870704
#SPJ11
A student wants to collect data during an experiment about the transfer of kinetic energy in a sample of water and ice. Which tool will help her collect the necessary data?
Answers
Given what we know, the tool in question that will help the student collect data regarding the transfer of kinetic energy between water and ice would be a thermometer.
How does the thermometer measure kinetic energy?It does not do so directly. However, kinetic energy in water molecules is reflected in the temperature of the water. When water molecules increase their kinetic energy and move more, they become hotter. Increased or decreased heat is an indirect way to measure the transfer of kinetic energy in water.
Therefore, given that the temperature of the water is a reflection of the transfer of kinetic energy happening, we can confirm that the tool that will help the student collect the data needed is a thermometer.
To learn more about kinetic energy visit:
https://brainly.com/question/999862?referrer=searchResults
Answer:
your answer will be a thermometer
Explanation:
if you go to connexus and are doing the science
Thermal Energy Unit Test 10 qestions 8th grd unit 5 test lesson 12 here are the correct answers
1. A. metal is heated from room temperature to 200'C.
2. B. The particles will have more space between them as steam, but they will be moving at the same speed in both states.
3. C. The potential energy decreases due to the tighter arrangement of the particles.
4. B. an increase in heat and an increase in kinetic energy until a phase change occurs.
5. C. thermometer
6. B. 4.
7. D. convection
8. C. Air moves from the areas of higher temperature to areas of lower temperature.
9. C. The temperature of the ice increases, while the temperature of the water decreases
10. A. heat transfer by radiation.
i hope this helps and please let me know if it does! = ) <3