Right after eating, all organs carry out glycolysis, ____ , and the ___
Answers
Right after eating, all organs carry out glycolysis, citric acid cycle , and the electron transport chain (ETC).
After eating, your body undergoes several metabolic processes to break down the food and produce energy. One of these processes is glycolysis, which is the breakdown of glucose (a sugar molecule) into two molecules of pyruvate. Glycolysis occurs in the cytoplasm of all organs and provides a quick energy source.
Another important process that follows glycolysis is the citric acid cycle, also known as the Krebs cycle or the tricarboxylic acid (TCA) cycle. The citric acid cycle is an aerobic pathway that takes place in the mitochondria of cells. The pyruvate molecules produced during glycolysis are converted into a molecule called acetyl-CoA, which then enters the citric acid cycle. Through a series of chemical reactions, the citric acid cycle generates energy in the form of adenosine triphosphate (ATP), as well as carbon dioxide and water as waste products.
Lastly, the electron transport chain (ETC) is another crucial process in cellular respiration. The ETC is located in the inner mitochondrial membrane and uses the high-energy electrons from the citric acid cycle to produce a proton gradient across the membrane. This gradient powers the synthesis of ATP via oxidative phosphorylation, providing the majority of the cell's energy needs.
In summary, after eating, all organs carry out glycolysis to produce pyruvate, which then enters the citric acid cycle and the electron transport chain to generate ATP, providing the necessary energy for various cellular processes.
Learn more about glycolysis here: https://brainly.com/question/28874793
#SPJ11
Related Questions
C1 JUN 22 Q4
Hydrazine can be manufactured from ammonia.
2NH₂ + H₂O₂ -
Calculate the atom economy of this reaction.
N₂H₂ + 2H₂O
atom economy -
calcium mass=
[1]
I
E
C1 JUN 22 Q7
Calculate the mass of calcium that contains the same number of atoms as there are molecules
in 9.1g of sulfur dioxide, SO₂
[2]
%
9
Answers
Answer:
[1]
The balanced chemical equation for the reaction is:
2NH₂ + H₂O₂ → N₂H₄ + 2H₂O
The molar mass of NH₂ is 16 g/mol (2 × 1.008 + 2 × 14.01), and the molar mass of H₂O₂ is 34 g/mol (2 × 1.008 + 2 × 16.00). The molar mass of N₂H₄ is 32 g/mol (2 × 14.01 + 2 × 1.008), and the molar mass of H₂O is 18 g/mol.
The total mass of the reactants is:
2 × 16 g/mol NH₂ + 34 g/mol H₂O₂ = 66 g/mol
The total mass of the products is:
32 g/mol N₂H₄ + 2 × 18 g/mol H₂O = 68 g/mol
The atom economy is the mass of the desired product divided by the total mass of the reactants, expressed as a percentage:
Atom economy = (mass of desired product / total mass of reactants) × 100%
Atom economy = (32 g/mol N₂H₄ / 66 g/mol) × 100%
Atom economy = 48.5%
To calculate the mass of calcium that contains the same number of atoms as there are molecules in 9.1 g of SO₂, we need to first find the number of molecules of SO₂ in 9.1 g of the compound.
[2]
The molar mass of SO₂ is 64 g/mol (32.06 + 2 × 16.00). The number of moles of SO₂ in 9.1 g of the compound is therefore:
n = mass / molar mass = 9.1 g / 64 g/mol = 0.142 mol
One mole of any substance contains the same number of particles (atoms, molecules, or ions) as the number of atoms in 12 g of carbon-12, which is approximately 6.02 × 10²³ particles (Avogadro's number). Therefore, the number of molecules of SO₂ in 9.1 g of the compound is:
number of molecules = n × Avogadro's number = 0.142 mol × 6.02 × 10²³/mol = 8.56 × 10²² molecules
To find the mass of calcium that contains the same number of atoms as there are molecules in 9.1 g of SO₂, we need to calculate the number of atoms in 8.56 × 10²² molecules of SO₂. The balanced chemical equation for the formation of SO₂ is:
S + O₂ → SO₂
One molecule of SO₂ contains one sulfur atom and two oxygen atoms. Therefore, 8.56 × 10²² molecules of SO₂ contain:
8.56 × 10²² molecules SO₂ × 1 sulfur atom / molecule = 8.56 × 10²² sulfur atoms
8.56 × 10²² molecules SO₂ × 2 oxygen atoms / molecule = 1.71 × 10²³ oxygen atoms
To obtain the same number of atoms of calcium, we need to divide the number of atoms of sulfur by the number of atoms of calcium in the following balanced chemical equation:
Ca + S → CaS
One calcium atom reacts with one sulfur atom to form one molecule of CaS. Therefore, the number of calcium atoms required is:
8.56 × 10²² sulfur atoms / 1 sulfur atom per Ca atom = 8.56 ×
In the lab, a student collects hydrogen gas over water in a eudiometer. The hydrogen gas is produced when a piece of magnesium metal reacts with excess hydrochloric acid. Part 1: (a) Write the balanced chemical equation for this reaction. Include the states of matter. Mg(s) + 2HCl (aq) — H2 (8) +MgCl, (aq) Part 2 out of 2 (b) How many moles of hydrogen gas are collected if 3.09 g of magnesium metal is used in the reaction? Report prc Moles of hydrogen gas mol H2
Answers
Moles of Hydrogen gas collected : 0.127
Further explanationThe reaction coefficient in a chemical equation shows the mole ratio of the components of the reactants and products
If one mole of the reactant or product is known, then we can determine the moles of the other compounds involved in the reaction
Reaction
Mg(s)+2HCl(aq)⇒H₂(g)+MgCl₂(aq)
mass of Mg=3.09 g
mol Mg (Ar= 24,305 g/mol) :
\(\tt mol=\dfrac{mass}{Ar}\\\\mol=\dfrac{3.09}{ 24,305}\\\\mol=0.127\)
Magnesium metal reacts with excess Hydrochloric acid, so Mg as a limiting reactant and moles of product is based on moles of Mg
From the equation, moles ratio Mg : H₂ = 1 : 1, so moles H₂ :
\(\tt \dfrac{1}{1}\times 0.127=0.127\)
(0)
Calculate the standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K.
Give your answer in kJ/mol
_________________________
Answers
The standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K is -0.956 kJ/mol.
Given: ΔT = 15 K and ∆K/K = 1/2
Temperature is directly proportional to equilibrium constant K,
So, ∆T/T = ∆K/K
This can be written as ∆K/K = ΔH°/R × (1/T2 − 1/T1)
On solving this equation, we getΔH° = −2.303 × R × ΔK/K × T2T2 = 310 + 15 = 325 K∆K/K = 1/2∆H° = −2.303 × 8.314 J mol−1 K−1 × 1/2 × 325 K∆H° = −955.7 J mol−1= −0.956 kJ/mol
Therefore, the standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K is -0.956 kJ/mol.
Learn more about enthalpy with the given link,
https://brainly.com/question/14047927
#SPJ11
8. Why are we focusing on high levels of ozone in the troposphere in this lab while in Lab 2 (Stratospheric Ozone) we focused on low concentrations of ozone in the stratosphere?
Answers
Answer:
f4
Explanation:
your welcome.
a patient's urine sample has a density of 1.02 g/ml. if 1250 ml of urine was excreted by the patient in one day, what mass of urine was eliminated?
Answers
Density is defined as the mass of a substance per unit volume. In this case, the density of urine is given as 1.02 g/ml. This means that for every 1 ml of urine, there is 1.02 g of mass.
To find the mass of urine eliminated by the patient in one day, we need to multiply the volume of urine by its density. The volume of urine is given as 1250 ml.
Mass of urine = Volume of urine x Density of urine
Mass of urine = 1250 ml x 1.02 g/ml
Mass of urine = 1275 g
Therefore, the mass of urine eliminated by the patient in one day is 1275 g.
Learn more about volume here
https://brainly.com/question/1578538
#SPJ11
is hydrogen monoxide covalent or ionic
Answers
Answer:
ionic
Explanation:
i just know i ❤️❤️]
❤their you go i hope it helps and i'm single looking for a friend sooooooooooooooooooo if you know what i mean just leave me a text
.
a commercial process for preparing ethanol (ethyl alcohol), , consists of passing ethylene gas, , and steam over an acid catalyst to speed up the reaction. the gas phase reaction is
Answers
The gas phase reaction for the commercial process of preparing ethanol (ethyl alcohol) involves the reaction between ethylene gas (C2H4) and steam (H2O) over an acid catalyst.
This process is known as the hydration of ethylene.
The reaction can be represented by the following equation:
C2H4 + H2O → C2H5OH
In this reaction, ethylene gas and steam combine to form ethanol. The acid catalyst, which is often a solid acidic material such as phosphoric acid or zeolite, helps to accelerate the reaction by providing a suitable environment for the chemical transformation.
The acid catalyst facilitates the protonation of the ethylene molecule, making it more susceptible to nucleophilic attack by the hydroxide ion derived from water. This leads to the formation of a carbocation intermediate, which is then further attacked by water, resulting in the formation of ethanol.
The gas phase reaction is preferred in this commercial process due to its higher efficiency and better control over the reaction conditions. By passing ethylene gas and steam over the acid catalyst, the reaction can be carried out at elevated temperatures and optimized reaction conditions to maximize the yield of ethanol.
In conclusion, the gas phase reaction for preparing ethanol involves the hydration of ethylene by steam in the presence of an acid catalyst.
Know more about Acid Catalyst here:
https://brainly.com/question/31671681
#SPJ11
Do you think the substances after the reaction was still copper (II) chloride and aluminum?
Answers
When aluminum is reacts copper (II) chloride then it will form aluminum chloride and Copper.
Aluminum as well as copper(II) chloride combine very vigorously, causing the reaction mixture to become extremely hot as heat was produced, the aluminum foil to breakdown, a reddish brown solid to appear, as well as gas bubbles to be released.
The chemical reaction can be written as:
2Al + 3CuCl2 → 3Cu + 2AlCl3
Therefore, Copper and aluminum chloride will be formed after the reaction as a product.
To know more about aluminum
https://brainly.com/question/28488595
#SPJ1
Why does the sun appear very large compared to the other stars
The other stars are much smaller than our sun.
O The sun is actually the largest star in the universe.
The sun is so bright that it appears larger than the other stars.
The other stars are much farther away from Earth than our sun.
Answers
Answer:
the other stars are much farther away from Earth than our sun
can you help me match these
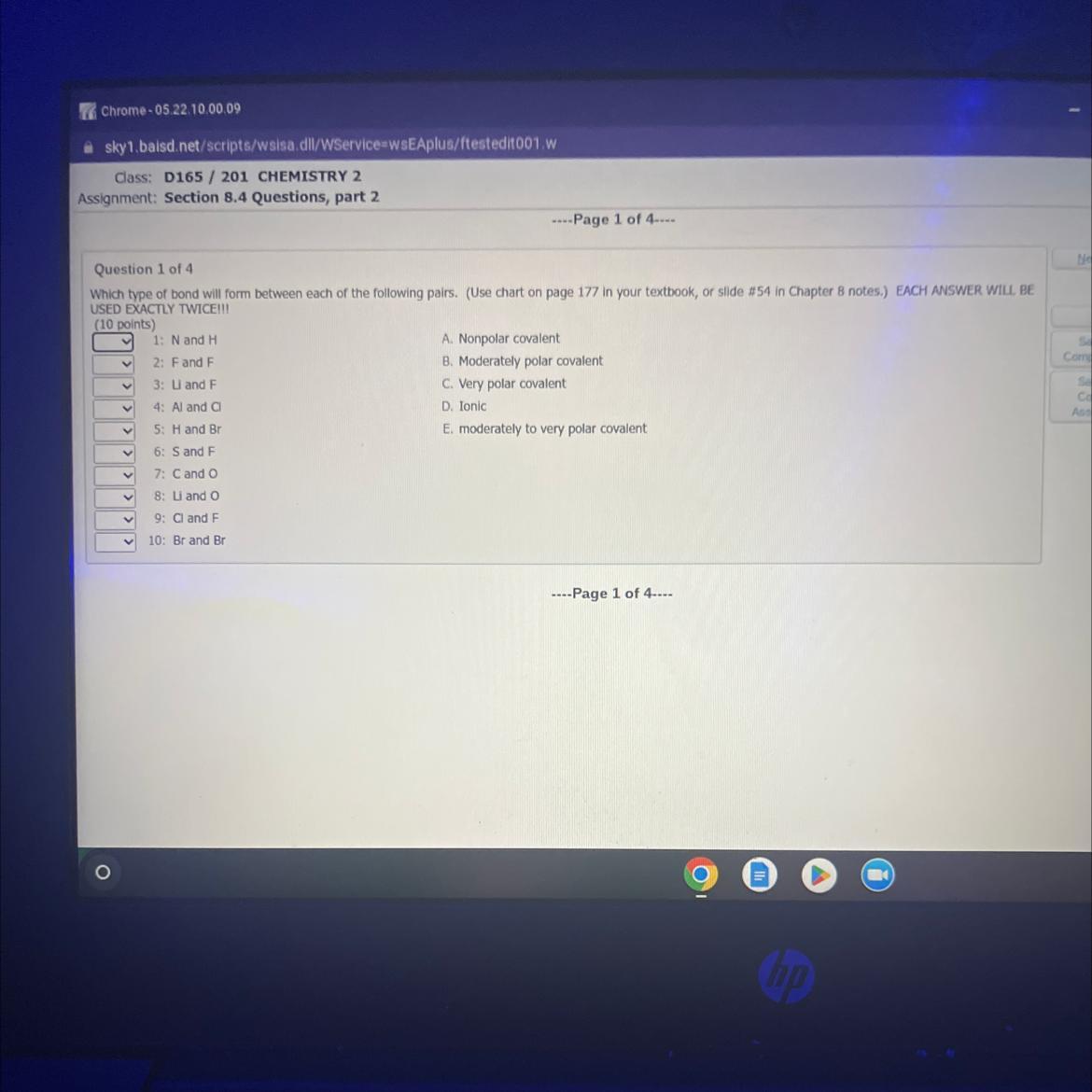
Answers
The following pairs are showing the types of bonds as follows:
1: N and H - Moderately to very polar covalent
2: F and F - Nonpolar covalent
What are the types of chemical bond ?An enduring attraction between atoms or ions known as a chemical bond is what allows molecules and crystals to form.
Ionic, covalent, hydrogen, and van der Waals interactions are the four types of chemical bonding that are necessary for life to exist.
3: Li and F - ionic bond
4: Al and Cl - Very polar covalent
5: H and Br - Moderately polar covalent
6: S and F - Very polar covalent
7: C and O - Moderately to very polar covalent
8: Li and O - Ionic
9: Cl and F - Moderately polar covalent
10: Br and Br - Nonpolar covalent
To learn more about the chemical bond, follow the link;
https://brainly.com/question/15444131
#SPJ9
Which word equation is the correct translation of the chemical reaction below?

Answers
Answer: We can translate the given chemical equation as:
"potassium permanganate combined with zinc chloride produces zinc permanganate and potassium chloride". The best option to answer the question is the first one.
Explanation:
The question requires us to choose, among the options given, the correct "translation" for the following chemical equation:
\(KMnO_4+ZnCl_2\rightarrow Zn(MnO_4)_2+KCl\)To solve this problem, we can separate the compounds into their cations and anions, and then analyze the metal (cation) name and anion name. Keep in mind that the name of the compound is formed by cation/metal name + anion name.
1) Let's start with KMnO4, which is formed by the cation K+ (potassium) and anion (MnO4)- (permanganate). The name of KMnO4 is potassium permanganate.
2) Next, let's analyze ZnCl2, which is formed by the cation Zn2+ (zinc) and anion Cl- (chloride). The name of ZnCl2 is zinc chloride.
3) Now, let's check Zn(MnO4)2, which is formed by Zn2+ (zinc) and (MnO4)- (permanganate). The name of Zn(MnO4)2 is zinc permanganate.
4) At last, let's analyze KCl, which is formed by K+ (potassium) and Cl- (chloride). The name of KCl is potassium chloride.
Therefore, we can translate the given chemical equation as:
"potassium permanganate combined with zinc chloride produces zinc permanganate and potassium chloride". The best option to answer the question is the first one.
Which statement best explains how the passage fits
the realistic fiction genre?
Answers
Answer:
It seems believable and familiar.
Explanation:
Plz mark Brainlest
calculate the [zn2 ] in a solution which is initially 030 m in [zn(cn)4]2- ions at 25
Answers
The concentration of Zn²⁺ ions in the solution at equilibrium is 4.59 x 10⁻¹⁹ M.
To calculate the concentration of Zn²⁺ ions in a solution that is initially 0.30 M in [Zn(CN)₄]²⁻ ions, we need to use the equilibrium constant expression for the dissociation of [Zn(CN)₄]²⁻ to Zn²⁺ and CN⁻ ions:
[Zn²⁺][CN⁻]⁴ / [Zn(CN)₄]²⁻ = K
where K is the equilibrium constant for the reaction.
At equilibrium, the concentration of [Zn(CN)₄]²⁻ will be equal to the initial concentration minus the concentration of Zn²⁺ ions that have been produced by dissociation, as [Zn(CN)₄]²⁻ dissociates to produce one Zn²⁺ ion and four CN⁻ ions:
[Zn(CN)₄]²⁻ → Zn²⁺ + 4CN⁻
Let x be the concentration of Zn²⁺ ions that have been produced at equilibrium. Then, the concentration of [Zn(CN)₄]²⁻ at equilibrium is (0.30 - x) M, and the concentration of CN⁻ ions is 4x M. Substituting these values into the equilibrium constant expression, we get:
[Zn²⁺][CN⁻]⁴ / [Zn(CN)₄]²⁻ = K
x(4x)⁴ / (0.30 - x) = K
Simplifying this equation and solving for x, we get:
x = [Zn²⁺] = K * (0.30 - x) / 256
Plugging in the given value of K and solving the equation, we get:
x = [Zn²⁺] = (1.2 x 10⁻¹⁶) * (0.30 - x) / 256
x = [Zn²⁺] = 4.59 x 10⁻¹⁹ M
Therefore, the concentration of Zn²⁺ ions in the solution at equilibrium is 4.59 x 10⁻¹⁹ M.
At 25°C, zinc cyanide has low solubility and may hydrolyze to form insoluble zinc hydroxide or zinc carbonate. Therefore, this calculation assumes that the solution is well-buffered to maintain the pH, and that no other factors such as precipitation or hydrolysis affect the concentration of Zn²⁺ ions in the solution.
To know more about concentration, refer to the link below:
https://brainly.com/question/30724941#
#SPJ11
Which statement correctly compares the number of protons and electrons in atom of rubidium (Rb) and Calcium (Ca)?
Answers
The question is incomplete, the complete question is;
Which statement correctly compares the number of protons and electrons in atoms of rubidium (Rb) and calcium (Ca)?
Rubidium has fewer protons and fewer electrons than calcium.
Rubidium has fewer protons and more electrons than calcium.
Rubidium has more protons and fewer electrons than calcium.
Rubidium has more protons and more electrons than calcium.
Rubidium has 37 electrons and protons as well as 48 neutrons. Calcium has 20 electrons, protons and neutrons.
Rubidium is a group 1 element while calcium is a group 2 element. Rubidium has more protons and more electrons than calcium.
Rubidium is an element in group one of the periodic table. It has 37 electrons and protons as well as 48 neutrons.
Calcium is an element in group two of the periodic table. It has Calcium has 20 electrons, protons and neutrons.
From the foregoing, we can see that Rubidium has more protons and more electrons than calcium hence the answer.
Learn more; https://brainly.com/question/7373020
Which landform explains the lack of cultural interaction between ancient China and ancient India? (4 points)
the Loess Plateau
the Chang River valley
the Gobi Desert
the Himalaya Mountains
Answers
Answer:
I had this question and it was the 4th one for me
Explanation:
What is the three carbon alcohol that forms the backbone of a triglyceride called?
Answers
The three carbon alcohol that forms the backbone of a triglyceride is called glycerol.
Glycerol is a type of alcohol that is found in many fats and oils. It is a clear, odorless, viscous liquid that is sweet-tasting and non-toxic. Glycerol is a versatile molecule that is used in a variety of industrial and pharmaceutical applications.
The structure of a triglyceride consists of three fatty acids that are linked to a glycerol molecule. The fatty acids are long-chain hydrocarbons that have a carboxylic acid group (-COOH) at one end. The carboxylic acid group can react with the hydroxyl group (-OH) on the glycerol molecule to form an ester linkage (-COO-). The resulting molecule is a triglyceride. Triglycerides are a type of lipid that are important for energy storage in the body. They are found in adipose tissue and are broken down into fatty acids and glycerol when the body needs to use stored energy.
Learn more about Glycerol here: https://brainly.com/question/23503113
#SPJ11
2. What happens when hydrochloric acid (HCl) is added to the solution? Do the relative concentrations of H+, CH3COOH, or
CH3C00 change when HCl is added to the solution?
Answers
Answer: A molecule of hydrochloric acid, for example, is composed of a hydrogen atom and a chlorine atom. When these molecules dissolve into water, they separate into a positively charged hydrogen ion and a negatively charged chlorine ion. ... Only some of the molecules of weak acids disassociate when added to water.
Explanation:

Question 3 (1 point)
Which of the parts of an atom can be different between atoms of the same element,
creating isotopes?
Electron
Neutron
12
Nucleus
Proton
please help!
Answers
Answer:
Neutron and Nucleus
Explanation:
Because isotopes are each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei.
Which characteristic allows a gas to be compressed?
OA. Its particles are in constant contact.
OB. Its particles have little energy.
OC. Its particles are spread far apart.
OD. Its particles have no motion.
Answers
Because there is so much empty space between the gas particles, most of a gas' volume is made up of compressible gas molecules, Hence option c is correct. i.e, Its particles are spread far apart.
The greater compressible nature of gases compared to liquids or solids is explained by the kinetic-molecular hypothesis. The average distance between the gas molecules at standard pressure and room temperature is roughly 10 times the diameter of the molecules. Gas particles are pushed closer together when a gas is compressed, such as when the diving tank is then being filled.
There are various uses for compressed gases. Patients with damaged lungs are frequently given oxygen in hospitals to help them breathe better. When a patient is having a major procedure, a compressed gas is usually used to give anesthetic.
To know more about compressibility of a gas, please refer:
https://brainly.com/question/1307771
#SPJ1
Which word describes the amount of matter an object contains?
O altitude
Odensity
O mass
O pressure
Answers
Thomas sits half way down a grassy slope. What force stopped him slamming down
Answers
Chlorine and hydrogen are combined and exposed to direct sunlight. Once exposed the chlorine and hydrogen explode and produce hydrogen chloride gas.
a. Rewrite the chemical reaction as a word equation
b. State the evidence that a chemical reaction has occurred
c. Is the reaction exothermic or endothermic? Explain your reasoning.
Answers
a. The chemical reaction can be rewritten as a word equation as follows:
Chlorine + Hydrogen -> Hydrogen Chloride + Explosion
b. Evidence that a chemical reaction has occurred includes the production of a new substance (hydrogen chloride gas), the release of energy (explosion), and a change in the physical properties of the reactants (change in color, odor, or temperature).
c. The reaction is likely exothermic, which means it releases energy. This is because the reaction produces a new substance (hydrogen chloride gas) and releases energy in the form of an explosion. In an endothermic reaction, the reactants would absorb energy from the surroundings.
How many weeks are equal to 2. 94x10^6 minutes?
Answers
To determine how many weeks are equal to 2.94x10^6 minutes, we can divide the total number of minutes by the number of minutes in a week. There are 60 minutes in an hour, and 24 hours in a day. Therefore, there are 60 x 24 = 1440 minutes in a day.
To find the number of minutes in a week, we multiply the number of minutes in a day by 7: 1440 x 7 = 10080 minutes.
Now, we can divide the total number of minutes (2.94x10^6) by the number of minutes in a week (10080) to find the equivalent number of weeks:
2.94x10^6 / 10080 = 291.66666667 weeks.
Since we cannot have a fraction of a week, we can round the answer to the nearest whole number. Therefore, 2.94x10^6 minutes is approximately equal to 292 weeks.
In summary, 2.94x10^6 minutes is equivalent to approximately 292 weeks.
learn more about weeks
https://brainly.com/question/25511600
#SPJ11
What is the movement of air parallel to Earth's surface called?
A. the Coriolis effect
B. windchill
C. wind
D.air pressure
Answers
Answer:
Wind is the movement of air parallel to Earth's surface.
Which set of numbers will balance the following equations? 1's have been included for clarity.__Mn3N4 + __NaF --> __MnF4 + __Na3N a 1; 4; 1; 4 b 1; 4; 3; 2 c 1; 12; 3; 4 d 3; 2; 3; 2
Answers
ANSWER
Option C
EXPLANATION
Given that;
\(\text{ ----- Mn}_3N_4\text{ }+\text{ ---- NaF }\rightarrow\text{ ---- MnF}_4\text{ }+\text{ ---Na}_3N\)In the reaction above, we have the following data
At the reactants side;
3 atoms of manganese
4 atoms of nitrogen
1 atom of sodium
1 atom of fluorine
At the products side
1 atom of manganese
4 atoms of fluorine
3 atoms of sodium
1 atom of nitrogen
To balance the above equation, apply the law of conservation mass
Law of conservation of mass states that matter can neither be created nor destroyed but can e transformed from one formato another.
To balance the equation, 1 mole of Mn3N4 reacts with 12 moles of Na Tto give 3 moles of MnF4 and 4 moles of Na3N
So, the new equation becomes
\(\text{ Mn}_3N_4\text{ }+\text{ 12NaF }\rightarrow\text{ 3MnF}_4\text{ }+\text{ 4Na}_3N\)The following data can be deduced in the above equation
At the reactants side
3 atoms of Mn
4 atoms of N
12 atoms of Na
12 atoms of F
At the products side
3 atoms of Mn
12 atoms of F
12 atoms of Na
4 atoms of N
Looking atthe vabove data, the number of atoms of each element at the reactants side is equal to the number of atoms of same elements at the products side.
Hence, the correct answer is option Ce
u
this question(s) make me cry :'[ help

Answers
While the atomic mass is simply the sum of the protons and neutrons, the average atomic mass is a weighted average of all the isotopes.
The isotope symbol can be used to identify the element's most prevalent form.
What is the relative atomic mass?Atomic mass is connected to mass number and average atomic mass, however their definitions differ.
The total number of protons and neutrons in an atom's nucleus is known as mass number. Usually, the letter A is used to denote it. Since the mass number is the sum of the whole numbers of protons and neutrons, it has an integer value.
Average atomic mass, on the other hand, is the weighted average of the masses of all the naturally occurring isotopes of an element, taking into account their abundance. It is usually represented by the symbol A or M.
Learn more about Atomic mass:brainly.com/question/11680817
#SPJ1
in one experiment, 0.886 mole of no is mixed with 0.503 mole of o2. determine which of the two reactants is the limiting reactant. calculate also the number of moles of no2 produced.
Answers
The balanced equation for the reaction between NO and O2 is given below;
2NO + O2 → 2NO2
The amount of NO used is greater than the amount of NO given.Therefore, O2 is the limiting reactant. The number of moles of NO2 produced is 0.0157 mol.
The balanced equation for the reaction between NO and O2 is given below;
2NO + O2 → 2NO2
Molar mass of NO = 30 g/mol
Molar mass of O2 = 32 g/molar
Calculate moles of NO and O2 in the reaction;
Moles of NO = Mass / Molar mass = 0.886 / 30 = 0.0295 mol
Moles of O2 = Mass / Molar mass = 0.503 / 32 = 0.0157 mol
b) Determine the limiting reactant;
To determine the limiting reactant, we compare the moles of the reactants with their coefficients in the balanced equation.
Moles of NO = 0.0295 mol
Coefficient of NO = 2
Moles of O2 = 0.0157 mol
Coefficient of O2 = 1
For NO,
Moles of NO used = 2 x Moles of O2 used = 2 x 0.0157 = 0.0314 mol
So, the amount of NO used is greater than the amount of NO given.Therefore, O2 is the limiting reactant.
c) Calculate the moles of NO2 produced;
The number of moles of NO2 produced is equal to the number of moles of the limiting reactant. Since the limiting reactant is O2,
Moles of NO2 = Moles of O2 = 0.0157 mol
Therefore, the number of moles of NO2 produced is 0.0157 mol.
To know more about limiting reactant visit:
https://brainly.com/question/10090573
#SPJ11
The volume of the compartment that holds this reaction inside your car's steering
column is approximately 0.050L before the airbag inflates.
a. Calculate the pressure in this compartment if it could hold the same number of moles of N 2 from above at a temperature of 45.0°C
b. Convert this pressure in kPa to atm. (Can you use this number in atm's to describe how many times greater than standard atmospheric pressure this
would be?)

Answers
a. The pressure in the compartment would be 6.7 atm at 45.0°C. b. Converting 6.7 atm to kPa gives 680.6 kPa. This is about 6.7 times greater than standard atmospheric pressure.
What is the volume of the compartment in m³?Converting 0.050 L to m³ gives 5.0 x 10^-5 m³.
If the temperature in the compartment was lowered to 20.0°C while the number of moles of N2 remained the same, what would be the new pressure in the compartment?Using the ideal gas law, we can calculate the new pressure to be 4.4 atm.
We can use the Ideal Gas Law to solve this problem, which states:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
a) We are given the volume V = 0.050 L, the temperature T = 45.0°C = 318.15 K, and the assumption that the compartment holds the same number of moles of N2 as in the previous question, which is n = 0.036 mol. We can rearrange the Ideal Gas Law to solve for P:
P = nRT/V
P = (0.036 mol)(0.0821 L·atm/mol·K)(318.15 K)/(0.050 L)
P = 146.7 atm
b) To convert this pressure to kPa, we can use the conversion factor 1 atm = 101.3 kPa:
P = 146.7 atm x 101.3 kPa/atm
P = 14862 kPa
To describe how many times greater than standard atmospheric pressure this would be, we can divide this pressure by standard atmospheric pressure, which is approximately 101.3 kPa:
14862 kPa / 101.3 kPa = 146.5
Learn more about pressure here:
https://brainly.com/question/12971272
#SPJ1
how many and bonds are in this molecule? the molecule n c c h o. note that there is a nitrogen carbon triple bond and a carbon oxygen double bond.
Answers
There are two bonds in the nitrogen-carbon triple bond and one bond in the carbon-oxygen double bond.
The molecule NCCCHO contains a nitrogen-carbon triple bond and a carbon-oxygen double bond. The nitrogen-carbon triple bond consists of two pi bonds and one sigma bond, for a total of three bonds. The carbon-oxygen double bond consists of one pi bond and one sigma bond, for a total of two bonds. Therefore, in total, there are five bonds in the molecule. The nitrogen-carbon triple bond is a strong bond due to the overlap of three hybridized orbitals, resulting in a shorter bond length and higher bond energy. The carbon-oxygen double bond is also strong, but less so than the nitrogen-carbon triple bond. The presence of these bonds affects the molecule's properties, such as its reactivity and polarity.
To know more about the pi bond visit:
https://brainly.com/question/17215490
#SPJ11
c. Complete the following combustion reaction. Write your balanced equation two different ways.
C8H18+O2→
C8H18+O2 →
Answers
The substance in reactants side shows a alkane which is burnt. As a result of combustion, Carbon dioxide and water is formed.
The equation will be like this:
\( \rm{ C_8H_18 + \dfrac{25}{2} O_2 =8 CO_2 + 9 H_2O }\)
Now here we can see that the stoichiometric coefficient is a fraction, hence Multiplying 2 in both sides of the eq.
\( \rm{2 C_8H_18 + 25 O_2 = 16 CO_2 + 18 H_2O }\)