Answers
Answer:
Question 1.
a. the product of the reaction above that contains calcium is calcium chloride, CaCl₂.
b. the reactant, hydrochloric acid, HCl contains hydrogen
c. the product water, H₂O contains hydrogen
d. the reactant, calcium carbonate, CaCO₃ contains carbon
e. The product carbon (iv) oxide contains carbon
Question 2:
a. atoms of hydrogen and oxygen bonded together as a single compound known as water molecules are present at the start of the reaction
b. two molecules of water are present at the start of the reaction
c. two individual atoms of hydrogen and oxygen existing as molecules are present at the end of the reaction.
d. at the end of the reaction, two molecules of hydrogen and one molecule of oxygen are present.
Question 3:
a. total mass of products = 187 g
b. if Chinua starts with 10 g of magnesium, the same 10 g of magnesium will be found in the magnesium sulfate
Explanation:
Question 1: The equation of the reaction between calcium carbonate and hydrochloric acid is given as follows: + 2HCl ---> CaCl₂ + H₂O + CO₂
a. the product of the reaction above that contains calcium is calcium chloride, CaCl₂.
b. the reactant, hydrochloric acid, HCl contains hydrogen
c. the product water, H₂O contains hydrogen
d. the reactant, calcium carbonate, CaCO₃ contains carbon
e. The product carbon (iv) oxide contains carbon
Question 2: the equation of the reaction in which water is broken down to hydrogen and oxygen is as follows: 2H₂O ----> 2H₂ + O₂
a. atoms of hydrogen and oxygen bonded together as a single compound known as water molecules are present at the start of the reaction
b. two molecules of water are present at the start of the reaction
c. two individual atoms of hydrogen and oxygen existing as molecules are present at the end of the reaction.
d. at the end of the reaction, two molecules of hydrogen and one molecule of oxygen are present.
Question 3: The equation of the reaction between magnesium and sulfuric acid is given as follows: Mg + H₂SO₄ ---> MgSO₄ + H₂
molar mass of Mg = 24.0 g; molar mass of H₂SO₄ = 98.0 g
a. when 37.0 g of magnesium is reacted with 150 g of H₂SO₄, the total mass of products of the reaction is equal to the total mass of the reactants according to the law of conservation of mass.
Total mass of products = 150 + 37 = 187 g
b. if Chinua starts with 10 g of magnesium, the same 10 g of magnesium will be found in the magnesium sulfate
Related Questions
How many moles of CaSO4 are required to produce 128 g of SO2? 3CaSO4 + CaS → 4CaO + 4SO2
Answers
2 moles of CaSO4 are required to produce 128 g of SO2 in 3CaSO4 + CaS → 4CaO + 4SO2 in this equation.
What do you mean by mole ?The term mole is defined as a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
1 mole = 6.023 × 10²³ molecules
The balance equation is as follows:
3CaSO4 + CaS → 4CaO + 4SO2
1 mole = 6.023 × 10²³ molecules
1 mole SO₂ = 6.023 × 10²³ molecules
128 mole = ?
Molar mass of SO₂ = 64 gram
The numbers of moles in 128 gram SO₂ = 1 / 64 ×128
= 2 moles
Thus, 2 moles of CaSO4 are required to produce 128 g of SO2.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
What is the mass in grams of 2.5 moles of Al?
Answers
Answer:
One mole of Al weighs 27g.
2.5 moles of Al weigh 67.5g.
What can the arrow in a chemical reaction be translated to mean? Check all that apply.
O yields
O accompanied by
O react to form
O added to
U except
Answers
The arrow in a chemical reaction can be translated to mean:
- O yields
- O react to form
Therefore, the correct options are "O yields" and "O react to form".
Choose the options below that are true.
A. The rate law for a given reaction can be determined from a knowledge of the rate-determining step in that reaction's mechanism.
B. The rate laws of all chemical reactions can be determined directly from their net chemical equations.
C. The rate laws of bimolecular elementary reactions are second order overall.
D. The rate law for a given reaction can be determined from its reaction mechanism, without the accompanying rates of each elementary step in the mechanism.
Answers
Answer:
The options (A) -The rate law for a given reaction can be determined from a knowledge of the rate-determining step in that reaction's mechanism. and (C) -The rate laws of bimolecular elementary reactions are second order overall ,is true.
Explanation:
(A) -The rate law can only be calculated from the reaction's slowest or rate-determining phase, according to the first sentence.
(B) -The second statement is not entirely right, since we cannot evaluate an accurate rate law by simply looking at the net equation. It must be decided by experimentation.
(C) -Since there are two reactants, the third statement is correct: most bimolecular reactions are second order overall.
(D)-The fourth argument is incorrect. We must track the rates of and elementary phase that is following the reaction in order to determine the rate.
Therefore , the first and third statement is true.
I need help figuring it out the answers were wrong I put in

Answers
a sample of ammonia has a mass of 45.5g. how many molecules are in this sample
Answers
Answer:
1.61 x 1024 molecules
Explanation:
See image below for step-by-step explanation

How many moles of H2 can be formed if a 4.71 g sample of Mg reacts with excess HCl?
Answers
Answer:
0.2 mole of H2.
Explanation:
We'll begin by calculating the number of mole in 4.71 g of magnesium (Mg). This can be obtained as follow:
Mass of Mg = 4.71 g
Molar mass of Mg = 24 g/mol
Mole of Mg =?
Mole = mass /Molar mass
Mole of Mg = 4.71 /24
Mole of Mg = 0.2 mole
Next, we shall write the balanced equation for the reaction.
This is illustrated below:
Mg + 2HCl —> MgCl2 + H2
From the balanced equation above,
1 mole of Mg reacted to produce 1 mole of H2.
Finally, we shall determine the number of mole of H2 produced from the reaction. This can be obtained as illustrated below:
From the balanced equation above,
1 mole of Mg reacted to produce 1 mole of H2.
Therefore, 0.2 mole of Mg will also react to produce 0.2 mole of H2.
Thus, 0.2 mole of H2 was obtained from the reaction.
The number of moles of H₂ that can be formed if a 4.71 g sample of Mg reacts with excess HCl is 0.19625 moles
Let's represent the reaction with a chemical equation
Mg + HCl → MgCl₂ + H₂
Mg + 2HCl → MgCl₂ + H₂
HCl is in excess according to the question. This means Mg is the limiting reagent and therefore determine the amount of product.
Therefore,
24 g of Mg gives 2g of H₂
4.71 g of Mg will give ? H₂
cross multiply
mass of H₂ = 4.71 × 2 / 24
mass of H₂ = 9.42 / 24 = 0.3925 g
moles of H₂ = mass / molar mass
moles of H₂ = 0.3925 / 2
moles of H₂ = 0.19625 moles
read more: https://brainly.com/question/18757768?referrer=searchResults
Choose the coefficient for oxygen, O2, which balances the following equation.
2P2O5 --> P4 + ?O2
Answers
Answer:
5
Explanation:
2P₂O₅=P₄+5O₂
How many grams of H2 would be formed if 34 grams of carbon reacted with an unlimited amount of H2O?
Answers
Answer:
The reaction between carbon (C) and water (H2O) forms carbon monoxide (CO) and hydrogen gas (H2). The balanced chemical equation for this reaction is:
C(s) + H2O(g) -> CO(g) + H2(g)
According to this balanced equation, one mole of carbon reacts with one mole of water to produce one mole of carbon monoxide and one mole of hydrogen gas.
First, calculate the number of moles of carbon in 34 grams. The molar mass of carbon is approximately 12.01 grams/mole.
Moles of carbon = 34 grams / 12.01 grams/mole = 2.831 moles
As the stoichiometry of the reaction shows a 1:1 ratio between carbon and hydrogen, the moles of hydrogen produced would also be 2.831 moles.
The molar mass of hydrogen (H2) is approximately 2 grams/mole.
So, the mass of hydrogen produced = 2.831 moles * 2 grams/mole = 5.662 grams
Therefore, if 34 grams of carbon reacts with an unlimited amount of water, approximately 5.66 grams of hydrogen gas would be formed.
Explanation:
Approximations followed for answer.
What happens when sodium and sulfur combine?
A) each sodium atom gains one electron
B) each sulfur atom loses one electron
C) each sodium atom loses one electron
D) each sulfur atom gains one electron
Answers
Answer:
BBBBBBBB ITS B
Explanation:
The reduction of iron(III) oxide () to pure iron during the first step of steelmaking, ()()() is driven by the high-temperature combustion of coke, a purified form of coal: ()()() Suppose at the temperature of a blast furnace the Gibbs free energies of formation of and are and , respectively. Calculate the maximum mass of pure iron that can be produced by the combustion of of coke. Round your answer to significant digits.
Answers
Answer:
hello your question is incomplete attached below is the complete question
answer : To 2 significant digits = 5500 kg
Explanation:
Given
C(s) + O2 (g) ----------> CO2 ( g )
mass of CO2 = 8.9 * 10^6 g
number of moles = 8.9 * 10^6 / 12 = 7.416 * 10 ^5 mol
same amount of moles is needed by O2 hence
attached below is the detailed solution

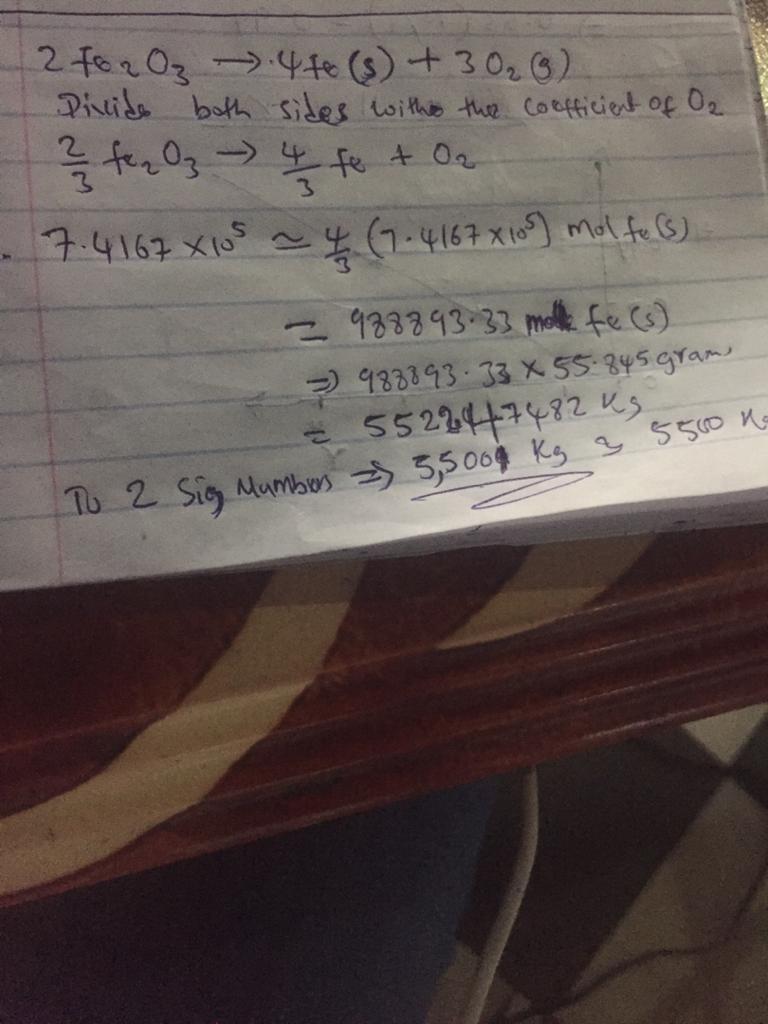
Which of the following has the greatest
effect on the ability of soil to hold water?
A. the age of the soil particles
B. the size of the soil particles
C. the color of the soil particles
D. the luster of the soil particles
Answers
The option that will have the greatest effect on the ability of soil to hold water is the size of the soil particles (option B).
What is water holding capacity?Soil water holding capacity is the amount of water that a given soil can hold for crop use. It is measured as the total quantity of water that can be absorbed by the soil per gram.
This characteristic is closely connected to the affinity that soil molecules have for water and other solutes in the environment.
The different soil types we have possess different water holding capacities. However, the size of each particle is a major determinant of this characteristics.
Learn more about water holding capacity at: https://brainly.com/question/28499548
#SPJ1
If 4.80 mol Ca mixed with 2 mol N2, which is the limiting reactant? 3Ca (s) + N2 (g) Ca3N2 (s)
Answers
Genetics and reproduction
Answers
genetics and reproduction is all about dna.
Balance the equations by putting the necessary coefficients in the blanks. Normally we do not write 1s when balancing, but for this particular question you need to include them for full credit. __Na3N___ Na +__ N2 ___H3PO4 + __ KOH __K3PO4 + __ H2O __ N2 +__ H2 __ NH3 __H2O2 __ O2 + __ H2O __ Zn + __ HCl __ ZnCl2 + __H2 __ C2H6 + __ O2 __ CO2 + __H2O __ CuCl2 + __H2S __ CuS + __HCl

Answers
Balancing a chemical equation is the process of ensuring that the number of atoms of each element in the reactants is equal to the number of atoms of that same element in the products.
Balance the chemical eqations given in the problem?
Na3N → 3 Na + ½ N2H3PO4 + 3 KOH → K3PO4 + 3 H2ON2 + 3 H2 → 2 NH3H2O2 → O2 + 2 H2OZn + 2 HCl → ZnCl2 + H2C2H6 + 7/2 O2 → 2 CO2 + 3 H2OCuCl2 + H2S → CuS + 2 HClChemical equations are used to describe the reactants and products in a chemical reaction. These equations are written using chemical formulas and symbols, indicating the types and numbers of atoms or molecules involved in the reaction. However, these equations must be balanced to obey the law of conservation of mass, which states that the total mass of the reactants must equal the total mass.
To learn more about chemical equation, visit: https://brainly.com/question/29886207
#SPJ1
What is the wavelength of a photon with a frequency of 6.56 x 10^14 Hz?
I will mark brainliest!!!!

Answers
Answer:457
Explanation:
How do you know your cell's are working
Answers
What results in the change of velocity?
Answers
Answer: An object can change velocity in a number of ways: it can slow down, it can speed up, or it can change direction. A change in speed, or a change in direction, or a change in both speed and direction means that the object has a change in velocity.
Explanation:
What would make oppositely charged objects attract each other more?increasing the positive charge of the positively charged object and increasing the negative charge of the negatively charged objectdecreasing the positive charge of the positively charged object and decreasing the negative charge of the negatively charged objectincreasing the distance between the positively charged object and the negatively charged objectmaintaining the distance between the positively charged object and the negatively charged object
Answers
Answer:
increasing the positive charge of the positively charged object and increasing the negative charge of the negatively charged object would make the oppositely charged objects attract each other more.
Lab: Types of Chemical Reactions
Student Guide
This laboratory allows you to study various kinds of chemical reactions, including some that result in precipitates.
Lesson Objectives
• Compare and contrast synthesis, single-displacement, and double-displacement reactions.
PREPARE
Approximate lesson time is 60 minutes.
Materials
• Lab Instructions: Lab_5.08_Instructions_modified_2020
• Lab Report: Lab_5.08_Report_modified_2020
• Lab Guidelines: Lab_Guidelines_modified
LEARN
Activity 1: Types of Chemical Reactions 1
Instructions
As you read through the lesson online, use the space below to take notes.
In this laboratory, you will study different kinds of chemical reactions.
Knowing the types of reactions helps you interpret your observations.
In a synthesis reaction, two reactants unite to form a third product.
In a single-displacement reaction, one ion of a reactant bonds with the second reactant.
In a double-displacement reaction, ions of both reactants change places.
Activity 2: Types of Chemical Reactions 1
Instructions
Procedure
1. Open the Chemical Reactions Virtual Lab.
2. Click View the Tutorial and complete the tutorial to learn how to conduct the lab.
3. Close the tutorial and click begin the Lab.
Part 1 Synthesis Reaction
4. Perform the procedure, placing the magnesium strip in the flame.
5. Record your reaction.
6. Research the chemical reaction of magnesium and oxygen gas. Write an equation for the chemical reaction
that accounts for the observed reaction in this part of the lab.
7. Answer the question: What is a synthesis reaction?
8. Answer the questions on Part 1 in the Lab Report.
Part 2 Single Displacement Reaction
9. Place 1 scoop of zinc in Vial A and add 10 drops of copper (II) sulfate. Observe the reaction.
10. Place ball of aluminum in Vial B and add 10 drops of copper (II) sulfate. Observe the reaction.
11. Place 1 scoop of zinc in Vial C and add 10 drops of silver nitrate. Observe the reaction.
12. Place copper wire in Vial D and add 10 drops of silver nitrate, wait 5 minutes. Observe the reaction.
13. Complete the ta
Answers
Answer:
hope this helps
Explanation:
see pictures try to put them in order




What is the name of the compound CaS? (5 points)
Calcium sulfur
Calcium sulfide
Calcium sulfite
Calcium sulfate
Answers
Answer: b) calcium sulfide
Explanation:
On average, about ____________ of incoming solar radiation is reflected back to space.
A 50%
B 30%
C 20%
D 10%
Answers
what is stoichiometry?
Answers
Answer:
the relationship between the relative quantities of substances taking part in a reaction or forming a compound, typically a ratio of whole integers.
Explanation:
G o o g l e
Explanation:
Stoichiometry is the calculation of reactants and products in chemical reactions in chemistry.
Seamus is conducting an experiment on electric force. He wants to get an approximate idea of how much force the charges will generate. Drag and drop the tiles to show the force of each situation in increasing order from lowest to highest (with repulsive forces being positive and attractive forces being negative).
=
One object with a charge of -4 × 10-5 C and another with a charge of 3 × 10-5 C placed 0.5
meters apart
One object with a charge of 3 x 10- C and another with a charge of -3 × 10-5 C placed 1
E
meter apart
= Two objects with a charge of 4 × 10-5 C placed 1 meter apart
= Two objects both with a charge of 3 × 10-5 C placed 0.5 meters apart
One object with a charge of 3 x 10- C and another with a charge of 4 x 10 C placed 1
E
meter apart

Answers
The highest electric force exerted by charges -4 ×10⁻⁵ C and 3 ×10⁻⁵ C placed 0.5 m apart is equal to 43.15 N.
The lowest electric force exerted by charges 3 ×10⁻⁵ C and 3 ×10⁻⁵ C placed 1 m apart is equal to 8.10 N.
What is coulomb's law?According to Coulomb’s law, the force of attraction between two charges is equal to the product of their charges and is inversely proportional to the square of the distance. This electric force applies along the line joining the two charges.
The magnitude of the electric force can be written as follows:
\(\displaystyle F = k\frac{q_1q_2}{r^2}\)
where k is constant proportionality = 8.99 × 10⁹ N.m²/C².
Given the charge on one point charge, q₁ = 4 ×10⁻⁵ C
The charge on the other point charge, q₂ = - 3 × 10⁻⁵C
The distance between these two charges, r = 0.5 m
The magnitude of electric force between the charges will be:
\(\displaystyle F = 8.99\times 10^{9}\times \frac{4\times 10^{-5}\times 3\times 10^{-5}}{(0.5)^2}\)
F = 43.15 N
Given the charge on one point charge, q₁ = 3 ×10⁻⁵ C
The charge on the other point charge, q₂ = 3 × 10⁻⁵C
The distance between these two charges, r = 1 m
The magnitude of force between the charges will be:
\(\displaystyle F = 8.99\times 10^{9}\times \frac{3\times 10^{-5}\times 3\times 10^{-5}}{(1)^2}\)
F = 8.1 N
Learn more about Coulomb's law, here:
brainly.com/question/506926
#SPJ1
How much potassium chloride will dissolve in 25 grams of water at 80°C?
Please I need help
Answers
Answer:
The problem provides you with the solubility of potassium chloride,
KCl
, in water at
20
∘
C
, which is said to be equal to
34 g / 100 g H
2
O
.
This means that at
20
∘
C
, a saturated solution of potassium chloride will contain
34 g
of dissolved salt for every
100 g
of water.
As you know, a saturated solution is a solution that holds the maximum amount of dissolved salt. Adding more solid to a saturated solution will cause the solid to remain undissolved.
In your case, you can create a saturated solution of potassium chloride by dissolving
34 g
of salt in
100 g
of water at
20
∘
C
.
Now, your goal here is to figure out how much potassium chloride can be dissolved in
300 g
of water at this temperature. To do that, use the given solubility as a conversion factor to take you from grams of salt to grams of water
Which solids are insoluble in water.
Answers
Some types of solids that are insoluble in water are:
Metals. (most of them)Non-Metallic ElementsMetal OxidesSome Non-Metallic ElementsMetal Carbonates (most of them)Metal Sulfides (most of them)Salts (some of them)Which solids are insoluble in water?Many solids are insoluble in water, meaning they do not dissolve in water to a significant extent. Here are some examples of common solids that are generally insoluble in water:
Metals: Most metals, such as gold, silver, platinum, and copper, are insoluble in water.
Non-Metallic Elements: Many non-metallic elements, such as carbon (in the form of graphite or diamond), sulfur, phosphorus, and iodine, are insoluble in water.
Metal Oxides: Some metal oxides, particularly those of less reactive metals, are insoluble in water. Examples include aluminum oxide (Al2O3), iron(III) oxide (Fe2O3), and lead(II) oxide (PbO).
Metal Carbonates: Most metal carbonates are insoluble in water. Examples include calcium carbonate (CaCO3), lead(II) carbonate (PbCO3), and copper(II) carbonate (CuCO3).
Metal Sulfides: Many metal sulfides are insoluble in water. Examples include lead(II) sulfide (PbS), silver sulfide (Ag2S), and mercury(II) sulfide (HgS).
Insoluble Salts: Certain salts have limited solubility in water. Examples include silver chloride (AgCl), lead(II) iodide (PbI2), and calcium sulfate (CaSO4).
It's important to note that while these solids are generally insoluble in water, they may exhibit some solubility to a small extent. The solubility of a solid in water can vary depending on factors such as temperature, pressure, and the presence of other solutes.
Learn more about solubility:
https://brainly.com/question/23946616
#SPJ1
A pure substance is a single kind of matter. A(n) _________
is made up of two or more substances that are together in the same place, but each substance keeps its own properties.
Answers
A pure substance is indeed a single kind of matter with a uniform and definite composition. In contrast, a mixture is made up of two or more substances that are together in the same place, but each substance maintains its own properties.
Mixtures can be classified as either homogeneous or heterogeneous. A homogeneous mixture has a uniform composition throughout, meaning its components are evenly distributed and not easily distinguishable. Examples include solutions, such as saltwater or air.
On the other hand, a heterogeneous mixture has an uneven distribution of its components, and its individual substances can be easily identified. Examples include sand and water, or oil and water.
One key distinction between a pure substance and a mixture is that a pure substance has a fixed composition, while the composition of a mixture can vary. This means that mixtures can be separated into their individual components through physical processes like filtration, evaporation, or distillation, without undergoing any chemical changes.
In summary, a pure substance is a single type of matter with a definite and uniform composition, while a mixture consists of two or more substances that are combined in the same place, but each substance retains its individual properties. Mixtures can be homogeneous or heterogeneous and can be separated into their components through physical methods.
Know more about Mixtures here:
https://brainly.com/question/24647756
#SPJ11
How much heat must be added to a 34.2 g sample of aluminum in order to raise the temperature of the aluminum 34 oC? (The specific heat of Aluminum is 0.9 J/g oC)
Answers
The amount of heat required to raise the temperature of the 34.2 g sample of aluminum by 34 oC is 1043.52 J.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance. It is a physical property that determines the direction of heat flow between two objects or systems in contact with each other. Temperature is measured in degrees Celsius (°C) or Fahrenheit (°F), or in kelvin (K) in the International System of Units (SI).
The amount of heat (q) required to raise the temperature of a substance can be calculated using the formula:
q = m x c x ΔT
Where:
m = mass of the substance (in grams)
c = specific heat of the substance (in J/g oC)
ΔT = change in temperature (in oC)
Plugging in the values given:
m = 34.2 g
c = 0.9 J/g oC
ΔT = 34 oC
q = (34.2 g) x (0.9 J/g oC) x (34 oC)
q = 1043.52 J
Therefore, the amount of heat required to raise the temperature of the 34.2 g sample of aluminum by 34 oC is 1043.52 J.
Learn more about Temperature
brainly.com/question/26866637
#SPJ1
Question 4 (1 point)
If the decomposition of (NH4)2(CO3) is a first-order process with a rate constant of
0.196 s-1, how much ammonium carbonate would remain after 39.0 s, starting from
a concentration of 0.957 M?
Your Answer in units:
Answers
The final concentration of the reactant of a first order reaction can be determined from the rate constant equation. The concentration of ammonium carbonate after 39 s will be 0.003 M.
What is rate constant?Rate constant of a reaction is the rate of reaction when one molar concentration of the reactant is involved in the reaction. The expression for the rate constant k for first order reaction is :
k = 1/t ln (C0/Ct)
Where C0 be the initial concentration and Ct be the concentration after t seconds.
Given that C0 of ammonium nitrate = 0.957 M
rate constant = 0.196 /s
t = 39 s.
The concentration after 39 seconds is calculated as follows:
0.196 /s = 1/39s ln (0.957 M / Ct)
Ct = 0.957 / (ln⁻¹ (0.196 × 39))
= 0.003 M.
Therefore, the concentration of ammonium carbonate after 39 seconds will be 0.003 M.
To find more on rate constant, refer here:
https://brainly.com/question/20305871
#SPJ1
What did the Copernicus revolution demonstrate?

