Answers
Answer:
C
Explanation:S has less energy than P, and P has less energy than D and so on.
Related Questions
How are oceans and salt marshes different? B A Salt marshes have deeper water than oceans. Salt marshes have shallower water than oceans. Salt marshes have more organisms than oceans. D . Salt marshes have more wave action than oceans. С
Answers
Answer:
Salt marshes have shallower water than oceans
Explanation:
oceans go way deeper then salt marshes with the deepest part of thr ocean at 35,876ft deep
Answer: Salt marshes have shallower water than oceans.
Explanation: oceans are deeper
All matter on earth is made up of
Answers
All matter is made up of atoms, which are in turn made up of protons, neutrons and electrons.
Answer:
Atoms
Explanation:
Atoms make up the matter on Earth. If you'd like, I can go a bit deeper, because I'm learning this stuff.
If I'm wrong, I sincerely apologize!
Write the chemical formula for Potassium Nitride
Answers
Answer:
KNO lower case 2
Explanation:
PLS HELP!!
The average human will breathe in 1.2 million moles of oxygen atoms in their lifetime. What is the mass of this amount of oxygen in grams?
Answers
Answer:
19,199,280 grams
Explanation:
0.062502343837894 grams in one mole of oxygen
look at attachment please help with it, will mark brainliest <3 BUT ONLY IF CORRECT

Answers
Mass is the measure of amount of matter in an object, it does not change.
Mass does not change with gravity, it is the weight that changes with gravity, as weight = mass x gravity.
a 12.3 ev electron collides with a hydrogen atom at rest in its ground state, exciting the electron to a higher energy level (n increases). 1)what is the largest value of n that can be reached considering conservation of energy? n
Answers
The largest value of n that can be reached considering conservation of energy when a 12.3 eV electron collides with a hydrogen atom at rest in its ground state, exciting the electron to a higher energy level is n= 2.
When a hydrogen atom is excited, it absorbs a photon and one of the electrons goes to a higher energy level. When an electron in an excited atom returns to a lower energy state, it releases a photon with a specific wavelength.
The energy absorbed by the hydrogen atom when it is excited is given by:ΔE = Ei - Efwhere Ei is the energy of the initial state, and Ef is the energy of the final state.In this situation, the electron has an energy of 12.3 eV, which is greater than the ionization energy of the hydrogen atom (13.6 eV).
As a result, the hydrogen atom cannot be ionized, but the electron can be excited to a higher energy level using this energy. The maximum value of n that can be reached with this energy can be determined by equating the energy of the initial state to the sum of the ionization energy and the energy of the final state.
The energy difference can be computed as follows:ΔE = Ei - Ef= 12.3 eV - 10.2 eV= 2.1 eVwhere Ef is the final state energy, which corresponds to the energy of the nth state of the hydrogen atom.
When we solve for n, we get:
n² = ΔE/13.6 eV= 2.1 eV/13.6 eV= 0.154n = √(0.154)= 0.392
Therefore, the maximum value of n that can be reached is n= 2 (i.e., the second energy level).
To learn more about hydrogen atom:
https://brainly.com/question/29695801#
#SPJ11
Which two of the following phrases describing the synthesis of amino acids are accurate?
1. Lysine is an essential amino acid and is synthesized in the human boy by the transamination of alpha-ketoglutarate.
2. Synthesis of the amino acid alanine involves reduction (that is, the precursor is reduced) its degradation involves oxidation
3. The carbon sources for nonessential amino acid synthesis include metabolic pathway intermediates and alpah-keto acids.
4. The synthesis of alanine from pyruvate, an alpah-keto acid, involves the oxidation of pyruvate.
5. The carboxyl groups of all the non essential amino acis are derived from glutamate.
Answers
2,5 are the two phrases describing the synthesis of amino acids are accurate.
Alpha-ketoglutarate is transaminated in the human body to produce lysine, an important amino acid. The precursor is reduced during the amino acid alanine's synthesis, and oxidation occurs during alanine's breakdown.
The precursor is reduced during the amino acid alanine's synthesis, while oxidation occurs during alanine's breakdown. Metabolic pathway intermediates and alpha-keto acids are the carbon sources for the production of non-essential amino acids.
To learn more about amino acid please click on below link
https://brainly.com/question/28409615
#SPJ4
what is the total number of electron-pair domains (electron clouds) around the central atom in the sulfur tetrafluoride molecule? enter your answer as a numeral without leading or lagging spaces. g
Answers
Total number of electron-pair domains (electron clouds) around the central atom in the sulfurtetrafluoride molecule is five. which includes 4 bond pair 1 loan pair.
what is electron pair domain?An atom's electron domain is the number of lone pairs or chemical bond locations that surround it. It represents the number of locations expected to contain electrons. By knowing the electron domain of each atom in a molecule, you can predict its geometry.
“The electron geometry describes the spatial arrangement of a molecule’s bonds and lone pairs. VSEPR theory can be used to calculate electron geometry.”
The term electron geometry is the name of the electron pair/groups/domains on the central atom, whether they are bonding electrons or non-bonding electrons. Electron pairs are electrons that exist in pairs or bonds, as lone pairs or as a single unpaired electron. Because electrons are always in motion and their paths cannot be precisely defined, the electron arrangement in a molecule is described in terms of an electron density distribution.
learn more about electron pair domain:https://brainly.com/question/16940491
#SPJ4
100 POINTS AND BRAINLIEST

Answers
Answer:
please brainliest
Explanation:
the equation formula answer is \(x^{2} fxb\sqrt{3\)
Answer:
the equation formula answer is x2 fxb\(\sqrt{3}\)
When you sweat a lot the water content of your blood can drop. Your cells have to maintain a certain level of water and salt in order to function properly. Which systems work together to help maintain homeostasis.
Answers
Answer:
Explanation:
There are key organs and system that assist in ensuring the body maintains homeostasis in the case of water-salt balance.
The excretory system: When one sweats, a lot of water content of the blood can drop (this is what causes the individual to be thirsty). In order to maintain a certain water-salt balance, the excretory system passes some salt out of the body in the form of urine (this is the reason the urine is usually yellowish but small in volume during the summer).
Endocrine system: When water content of the blood drops, vasopressin (also called antidiuretic hormone) is released into the bloodstream stimulating the kidneys to conserve water and release less urine (as said earlier). However, if the body is hydrated, less vasopressin is released and more water is passed in the urine.
Since models are representations, they have limits on how precisely they describe reality. Consider your model. What approximations or assumptions does your model contain? How does each one limit your model’s explanatory power?
Answers
A model helps to explain a physical reality.
What are models?A model is a representation of reality. It often serves the purpose of explanation or prediction. The question is incomplete but I will try to explain what a model is.
There are several kinds of models such as;
Physical modelsGraphic modelsComputer models Mathematical modelsLearn more about models: https://brainly.com/question/731147
someone pls explain how I do this work
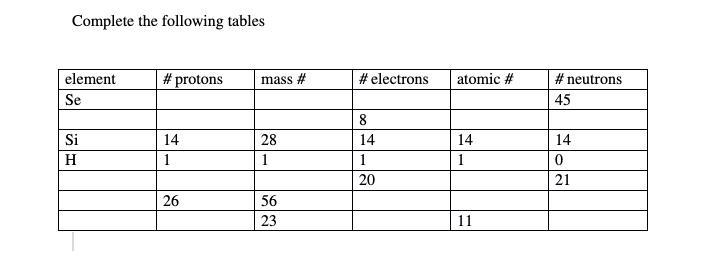
Answers
Answer:
Explanation:
Element #of protons Mass #. # of electrons Atomic #. Neutron
Se. 34. 78.96. 34 34 45
Si. 14. 28. 14. 14 14
H. 1. 1. 1 1 0
Ca. 20. 40.078. 20. 20. 21
Fe. 26. 56. 26. 26. 30
Na. 11. 23. 11. 11. 12
2. Why is it important in gravimetric analysis to add an excess amount of precipitating ions to a solution containing an analyte?
Answers
It is important in gravimetric analysis to add an excess amount of precipitating ions to a solution containing an analyte because it helps to determine the mass of the analyte.
What is Gravimetric analysis?This is a method which is used in the determination of the quantitative determination of the ion being analyzed(analyte) based on its mass.
Adding an excess amount of precipitating ions to a solution containing an analyte will make it easier for the mass of an analyte to be determined.
It is also important for the precipitate to be a pure substance with a definite and known composition which therefore makes it the most appropriate reason.
Read more about Gravimetric analysis here https://brainly.com/question/6163057
#SPJ1
Which of the following should have the lowest boiling point? A) C5H12 B) C6H14 C) C8H18 D) C10H22 E) C12H26
Answers
C₅H₁₂ has the lowest boiling point of all the given options. The correct answer is A.
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the external pressure surrounding the liquid.
The boiling point of a substance is affected by the strength of the intermolecular forces present in the substance. The stronger the intermolecular forces, the higher the boiling point.
The compounds in the question are all hydrocarbons. Hydrocarbons are nonpolar molecules and have London dispersion forces as their only intermolecular force.
The strength of London dispersion forces depends on the size of the molecule. The larger the molecule, the stronger the London dispersion forces.
The boiling point of a hydrocarbon increases with increasing molecular weight. The compound with the lowest molecular weight is C₅H₁₂, so it will have the lowest boiling point. Therefore, the correct answer is A, C₅H₁₂.
To know more about boiling point, refer here:
https://brainly.com/question/32297260#
#SPJ11
. Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
(a) 0.200 M HCl
(b) 0.0143 M NaOH
(c) 3.0 M HNO3
(d) 0.0031 M Ca(OH)2
Answers
pH= -log [H3O+]
PH=-log (0.200)
= 0.699
poH= 14-0.699
= 13.301
b. NaOH:
PoH= -log [OH-]
= -log (0.0143)
= 1.845
pH= 14-poH
= 14- 1.845
= 12.16
c. HNO3:
PH= -log[H3O+]
=-log(3.0)
= -0.4771
poH= 14-pH
= 14-9-0.4771
= 14.4771
pH= -0.4771, poH= 14.4771
d. [Ca(OH)2] = 0.0031M
[OH-]= 2X0.0031
[OH-] = 0.0062M
PoH= - log[OH-]
=-log(0.0062)
=-log(6.2x10-3)
=-(-2.21)
= 2.21
PH=14-poH
=14-2.21
=11.79
POH=2.21, PH= 11.79
Based on the molarity of the solutions;
For 0.200 M HCl; pH = 0.699, pOH = 13.301For 0.0143 M NaOH; pOH = 1.845, pH = 12.16For 3.0 M HNO3; pH = -0.4771, poH = 14.4771For 0.0031 M Ca(OH)2; pOH = 2.21, pH = 11.79What pHand pOH?pH is the negative logarithm to base ten of the hydrogen ions concentration of a solution.
pH = -log[H+]pOH is the negative logarithm to base ten of the hydroxide ions concentration.
pOH = -log[OH-]Also;
pH + pOH = 14For HCl:
pH = -log [H3O+]
pH =-log (0.200)
pH = 0.699
Then;
poH= 14-0.699
pOH = 13.301
For NaOH:
pOH= -log [OH-]
= -log (0.0143)
pOH = 1.845
Then;
pH= 14-poH
= 14- 1.845
pH = 12.16
For HNO3:
pH= -log[H3O+]
=-log(3.0)
= -0.4771
Then;
pOH = 14-9-0.4771
pOH = 14.4771
For [Ca(OH)2]
molarity = 0.0031M
2 moles of OH- are produced
[OH-]= 2 × 0.0031
[OH-] = 0.0062M
pOH = - log[OH-]
=-log(0.0062)
=-log(6.2x10-3)
=-(-2.21)
pOH = 2.21
Then;
pH =14-2.21
pH =11.79
Learn more about pH and pOH at: https://brainly.com/question/13557815
A solution has a concentration of 1.4 mol/dm3. How many moles of solute will there be in 750 cm3 of this solution?
Answers
Answer:
1.05 mol
Explanation:
c = number of mole of solute/volume of solution
1.4=n/750cm^3

Which element is number 14 on the periodic table?.
Answers
Answer:
Silicon
Explanation:
When viewing the periodic table of elements and locating atomic number 14, you will find that the element present is Silicon (Si), of which is within the metalloid elemental group.
Fe(s) + O2(g) →Fe₂O3(s)
Answers
Answer:
4Fe(s) + 3O₂(g) = 2Fe₂O₃(s)
A Gas Undergoes A Cycle In A Piston–Cylinder Assembly Consisting Of The Following Three Processes:
Answers
A piston–cylinder assembly is a device used to measure the pressure, volume, and temperature of a gas. It usually consists of a cylinder with a movable piston, which is used to compress or expand the gas within the cylinder. The three processes in a piston–cylinder assembly are usually compression, expansion, and isothermal processes.
In compression, the gas is compressed by the piston, decreasing the volume of the cylinder and increasing the pressure of the gas. Expansion is the opposite process, where the piston moves outward and increases the volume of the cylinder while decreasing the pressure of the gas. An isothermal process is a type of process where the temperature remains constant while the pressure and volume of the gas change.
These three processes can be combined to create a thermodynamic cycle. In this type of cycle, the gas undergoes a series of isothermal, expansion, and compression processes, which return the gas to its original state. This type of cycle is used to measure the thermodynamic properties of a gas, such as its heat capacity and efficiency.
Learn more about Cycle In A Piston–Cylinder:
https://brainly.com/question/13771818
#SPJ4
Will the electronegativity of barium be larger or smaller than that of strontium?
Answers
Answer:The electronegativity is smaller
Explanation:
The property of electronegativity is that it gets higher with going up and to the right on the periodic table. Barium is down and to the left of strontium, and thus, has less electronegativity
You can give brainliest if u want. :D :)
The electronegativity of barium (Ba) would be smaller than that of strontium (Sr).
Electronegativity refers to a measure of the ability of an atom of a chemical element to attract any shared pair of electrons.
On the periodic table, the electronegativity of a chemical element typically increases across the period from left to right and decreases as you move down a group.
Note: Barium (Ba) and strontium (Sr) are in the same group on the periodic table. Also, they are referred to as alkali earth metals because they all have two (2) valence electrons in their outermost shell.
Since barium (Ba) is found below strontium (Sr) on the periodic table, it would have a smaller electronegativity because it decreases as you move down a group.
Read more on electronegativity here: https://brainly.com/question/7970624
what is decomposition reaction
example
Answers
A decomposition reaction is a type of chemical reaction where a compound breaks down into two or more simpler substances. This process is typically induced by heat, light, or an electrical current.
In a decomposition reaction, the reactant compound typically breaks down into two or more products, which can be elements or simpler compounds.
There are various types of decomposition reactions, such as thermal decomposition, electrolytic decomposition, photolytic decomposition, and catalytic decomposition, depending on the type of energy that is used to initiate the reaction.
For example, the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2) is a decomposition reaction:
\(2H_2O_2 --- > 2H_2O + O_2\)
Thus, this is decomposition reaction.
For more details regarding decomposition reaction, visit:
https://brainly.com/question/16987748
#SPJ1
\( \huge \red {Answer} \)
A decomposition reaction is a type of chemical reaction where a compound breaks down into two or more simpler substances. This process is typically induced by heat, light, or an electrical current.In a decomposition reaction, the reactant compound typically breaks down into two or more products, which can be elements or simpler compounds.seawater has a density of about 1.03g/ml in temperate climates. if seawater contains 1.20mg of gold per ton of seawater, how many liters of seawater would it take to collect 1.00 kg of gold?
Answers
It would take approximately \(8.55 x 10^5\) liters of seawater to collect 1.00 kg of gold.
To solve this problem, we first need to determine the concentration of gold in seawater in units of g/L.
1 ton of seawater is equivalent to 1000 kg of seawater. If 1.20 mg of gold is present in one ton (1000 kg) of seawater, then the concentration of gold in seawater is:
\((1.20 mg/ton) / 1000 = 1.20 x 10^-6 g/kg\)
Next, we can set up a proportion to find the volume of seawater required to collect 1.00 kg of gold:
(concentration of gold) / (density of seawater) = (mass of gold) / (volume of seawater)
Plugging in the given values, we get:
\((1.20 x 10^-6 g/kg) / (1.03 g/mL) = (1000 g) / (volume of seawater)\)
Solving for the volume of seawater, we get:
volume of seawater = \((1000 g) / [(1.20 x 10^-6 g/kg) / (1.03 g/mL)] = 8.55 x 10^8 mL\)
Converting to liters, we get:
volume of seawater = \(8.55 x 10^5 L\)
Therefore, it would take approximately \(8.55 x 10^5\) liters of seawater to collect 1.00 kg of gold.
To know more about density refer to-
brainly.com/question/29775886#
#SPJ11
write the balanced net ionic equation for the reactions that occur when the given aqueous solutions are mixed. include the physical states. a. silver nitrate, agno3 , and magnesium bromide, mgbr2
Answers
A. AgBr = Ag+(aq) + Br(aq) (s)
B. H+(aq), OH(aq), and water (l)
C. NH4Cl + NH4+(aq) + Cl(aq) (s)
For the processes that take place when the two provided aqueous solutions are combined, we want to develop the balanced net ionic equations.
A net ionic equation: What is it?a chemical equation that just includes the ions going through chemical changes during the reaction.
A. Magnesium bromide, MgBr2, and silver nitrate, AgNO3.
AgBr = Ag+(aq) + Br(aq) (s)
Because they are spectator ions, NO3 and Mg2+ are excluded from the net ionic equation.
B. potassium hydroxide, KOH, and perchloric acid, HClO4. H+(aq) + OH(aq) H2O (l)
Because they are spectator ions, ClO4 and K+ are excluded from the net ionic equation.
To know more about ionic equation visit:-
https://brainly.com/question/15466794
#SPJ4
What is the physical state of oxygen at 1 atm of pressure?
Answers
Oxygen is indeed a gas under normal circumstances. However, the gas transforms into a liquid or a solid at low temperatures and/or high pressure. The air has 0.21 atm of oxygen in it.
What are the uses of oxygen?The energy-producing process that powers the metabolism rate of most living organisms, respiration, depends heavily on oxygen. All living things, including humans, depend on the air that we breath to survive.
How does oxygen become made?Oxygen is produced by cyanobacteria, algae, and plants. Photosynthesis is how they accomplish this. They convert both water and carbon dioxide create glucose and oxygen using the sunlight's energy. The sugars are used in their diet. Oxygen has small intermolecular forces.
To know more about oxygen visit:
https://brainly.com/question/11556976
#SPJ1
Suppose you mix 100.0 g of water at 25.5 oC with 75.0 g of water at 76.2 oC. What will be the final temperature of the mixed water, in oC
Answers
The final temperature of the mixed water will be approximately 47.23 °C.
To find the final temperature of the mixed water, we can apply the principle of conservation of energy, assuming no heat is lost to the surroundings.
The amount of heat gained by the cooler water is equal to the amount of heat lost by the hotter water.
The heat gained or lost by a substance can be calculated using the formula:
Q = m × c × ΔT
where Q is the heat gained or lost, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature.
Given:
Mass of the first water sample (m1) = 100.0 g
The temperature of the first water sample (T1) = 25.5 °C
Mass of the second water sample (m2) = 75.0 g
The temperature of the second water sample (T2) = 76.2 °C
Let's assume the final temperature of the mixed water is Tfinal.
The heat gained by the cooler water is:
Q1 = m1 × c × (Tfinal - T1)
The heat lost by the hotter water is:
Q2 = m2 × c × (T2 - Tfinal)
Since the amount of heat gained is equal to the amount of heat lost, we can set up the equation:
Q1 = Q2
m1 × c × (Tfinal - T1) = m2 × c × (T2 - Tfinal)
Now we can substitute the given values into the equation:
100.0 g × c × (Tfinal - 25.5) = 75.0 g × c × (76.2 - Tfinal)
Simplifying the equation:
100.0 × (Tfinal - 25.5) = 75.0 × (76.2 - Tfinal)
Expanding:
100.0 Tfinal - 2550 = 75.0 × 76.2 - 75.0 Tfinal
Combining like terms:
100.0 Tfinal + 75.0 Tfinal = 75.0 × 76.2 + 2550
175.0 Tfinal = 5715 + 2550
175.0 Tfinal = 8265
Dividing both sides by 175.0:
Tfinal = 8265 / 175.0
Tfinal ≈ 47.23 °C
Learn more about the final temperature at
https://brainly.com/question/2264209
#SPJ4
(Thermodynamics question) When dynamite and oxygen create an explosion, the q value for the system (reactants), would be __
a. positive
b. negative
c. zero
Answers
Below is the structure for the amino acid glycine. Which bond angles are closest to the actual values for the h-n-c and o-c-o bond angles? consider all lone pairs of electrons as substituents when answering this question.
Answers
We have that the bond angles that are closest to the actual values for the h-n-c and o-c-o bond angles are
109.5 degrees.120 degrees.H-N-C and O-C-O bond anglesGenerally, In H-N-C bond nitrogen has Sp3 hybridization, it will have the angle 109.5 degrees.
In O-C-O bond C atom has sp^2 Hybridization so it will have the angle by 120 degrees.
Therefore,bond angles that are closest to the actual values for the h-n-c and o-c-o bond angles are
109.5 degrees.120 degrees.For more information on Bonding visit
https://brainly.com/question/819068
When 0. 4g of zinc trioxocarbonate (IV) reacted with dilute hydrochloric acid, zin chloride wa produced and carbon (IV) oxide evolved if the reaction took 2minute. What wa the rate of the reaction
Answers
The rate of reaction when 0. 4g of zinc trioxocarbonate (IV) reacted with dilute hydrochloric acid, zin chloride was produced and carbon (IV) oxide evolved if the reaction took 2minute is 0.0016 mol/min
The rate of reaction is the time taken for product molecules to appear or the time taken for reactant molecule to be converted and the rate of reaction = mole of product formed/time taken
And the equation of the reaction is
ZnCO₃ + 2HCl → ZnCl₂ + H₂O + CO₂
And the molar mass of zinc carbonate = 125.39 = 8g/mol
Mole of zinc carbonate converted = 0.4/125.38
Time taken = 2 min
Rate of reaction = 0.0032/2
Rate of reaction = 0.0016 mol/min
Know more about reaction
https://brainly.com/question/29346459
#SPJ4
A 1.00 L solution saturated at 25∘C with lead(II) iodide contains 0.54 g of PbI2.
Calculate the solubility-product constant for this salt at 25∘C.
Answers
The solubility-product constant for this salt at \(25^{0}\)C is 6.4 × \(10^{-9}\)
The concentration of the ions in a saturated solution that are in equilibrium with the solid substance is known as the solubility product, or Ks, of an ionic compound.
The property that a material exhibits in the solute for getting dissolved in a solvent in order to form a solution is defined as the solubility product. The ionic chemicals that dissociate to form cations and anions in water vary more in terms of solubility.
Moles of dissolved \(PbI_{2}\) = 0.54 g /461.05 g/mol =0.00117
concentration of dissolved \(PbI_{2}\) = 0.00117 mol / 1.0 L = 0.00117 M
Concentration \(Pb^{2+}\) = 0.00117 M
Concentration \(I^{-}\) = 2 x 0.00117 =0.00234 M
\(K_{sp}\) =0.00117 \((0.00234)^{2}\) = 6.4 x \(10^{-9}\)
Learn more about solubility-product constant:
brainly.com/question/1419865
#SPJ4
The radius of an iron atom has been calculated to be about 0.00000000014 m. What is this length in scientific notation?Atom A and Atom B have the same number of protons and neutrons, but they do not have the same number of electrons.
Which statement describes the atoms?
A The atoms have the same chemical symbol.
B The atoms have the same charge.
C The atoms have different atomic numbers.
D The atoms have different atomic masses.
Answers
A. The atoms have the same chemical symbol.
