Answers
Answer:
We have,
Initial volume is V
1
Initial temprature is T
1
=75
0
C
Initial pressure is P
1
So, final temprature is T
2
=273+75=348K
V
2
=V
1
−
100
15
V
1
=
100
85V
1
Applying the gas formula,
T
1
P
1
V
1
=
T
2
P
2
V
2
348
P
1
V
1
=
T
2
×100
2P
1
×85V
1
T
2
=
100
2×85×348
T
2
=591.6K
Related Questions
A compound has a similar molecular mass of 180grams/mol. It contains 40.8% carbon,5.8% hydrogen and 53.4% oxygen. Calculate its empirical formula and molecular formula, and give its common name.
Answers
Answer:
To find the empirical formula of the compound, we need to determine the simplest whole-number ratio of atoms in the molecule.
First, we can assume that we have 100 grams of the compound, and calculate the number of moles of each element present in it:
Carbon: 40.8 g / 12.011 g/mol = 3.398 mol
Hydrogen: 5.8 g / 1.008 g/mol = 5.753 mol
Oxygen: 53.4 g / 15.999 g/mol = 3.337 mol
Next, we divide each of these values by the smallest one, which is 3.337, to get the mole ratio:
Carbon: 3.398 mol / 3.337 mol = 1.019
Hydrogen: 5.753 mol / 3.337 mol = 1.723
Oxygen: 3.337 mol / 3.337 mol = 1.000
Rounding these values to the nearest whole number, we get the empirical formula: C1.0H1.7O1.0, which can be simplified to CH1.7O.
To find the molecular formula, we need to know the actual molecular mass of the compound. We can estimate it by adding the atomic masses of the elements in the empirical formula:
Carbon: 1 x 12.011 = 12.011
Hydrogen: 1.7 x 1.008 = 1.714
Oxygen: 1 x 15.999 = 15.999
Total = 29.724 g/mol (approx.)
The molecular mass is close to 180 g/mol, which suggests that the actual molecular formula is a multiple of the empirical formula. Dividing 180 by 29.724, we get a value of about 6.05. Multiplying the subscripts in the empirical formula by this value, we get the molecular formula: C6H10.2O6, which can be further simplified to C3H5.1O3.
The compound with the empirical formula CH1.7O and the molecular formula C3H5.1O3 is commonly known as glyoxylic acid.
1. A sample of commercial concentrated hydrochloric acid is 11.8 M HCl and has a density of 1.190 g/mL. Calculate (a). the mass % of HCI (b). the molality of HCI (c). the mole fraction of HCI
Answers
(a) The mass percent of HCl in the solution is approximately 36.1%.
(b) The molality of HCl in the solution is approximately 15.5 mol/kg.
(c) The mole fraction of HCl in the solution is approximately 0.218.
(a) To calculate the mass percent of HCl, we need to determine the mass of HCl in a given volume of the solution.
Given: Concentration of HCl = 11.8 M
Density of the solution = 1.190 g/mL
First, we need to calculate the mass of the solution. Since density is mass per unit volume, the mass of 1 mL of the solution is 1.190 g.
Next, we need to calculate the mass of HCl in 1 mL of the solution. Since the concentration is given in moles per liter (M), and the molar mass of HCl is 36.46 g/mol, we can calculate the mass of HCl in 1 mL as follows:
Mass of HCl = concentration × volume × molar mass
= 11.8 mol/L × 0.001 L × 36.46 g/mol
= 0.430 g
Now, we can calculate the mass percent of HCl using the following formula:
Mass percent = (mass of solute ÷ mass of solution) × 100
= (0.430 g ÷ 1.190 g) × 100
≈ 36.1%
(b) The molality of HCl is calculated by dividing the moles of solute (HCl) by the mass of the solvent (water) in kilograms.
Since the density of the solution is given as 1.190 g/mL, the mass of 1 mL of the solution is 1.190 g. However, we need to consider the density of the solvent (water) to calculate the mass of water in the solution.
Assuming the density of water is 1 g/mL, the mass of water in 1 mL of the solution is (1.190 g - 0.430 g) = 0.760 g.
To calculate the molality of HCl, we need to convert the mass of water to kilograms:
Mass of water (kg) = 0.760 g ÷ 1000 = 0.000760 kg
The molality (m) is calculated using the formula:
Molality = (moles of solute ÷ mass of solvent in kg)
= (11.8 mol/L × 0.001 L) ÷ 0.000760 kg
≈ 15.5 mol/kg
(c) The mole fraction (X) of HCl is calculated by dividing the moles of HCl by the total moles of all components in the solution.
To calculate the mole fraction, we need to consider the volume of the solution and convert it to liters.
Given: Concentration of HCl = 11.8 M
Volume of the solution = 1 mL
Volume of the solution (L) = 1 mL ÷ 1000 = 0.001 L
To calculate the mole fraction of HCl, we need to calculate the moles of HCl and the moles of water (solvent) in the solution.
Moles of HCl = concentration × volume
= 11.8 mol/L × 0.001 L
= 0.0118 mol
Moles of water = mass of water ÷ molar mass of water
= 0.760 g ÷ 18.015 g/mol (molar mass of water)
= 0.0422 mol
Total moles in the solution = moles of HCl + moles of water
= 0.0118 mol + 0.0422 mol
= 0.054 mol
Mole fraction of HCl = moles of HCl ÷ total moles
= 0.0118 mol ÷ 0.054 mol
≈ 0.218
For such more questions on molality
https://brainly.com/question/14366957
#SPJ8
Which of the following describes an impact of the specific heat of water on the planet? (3 points)
A. Islands and coastal places have moderate pleasant climates.
B. Ocean waters experience sudden spikes and drops in temperature.
C. The internal temperature of living organisms varies over a wide range.
D. Inland places have minimal temperatures changes throughout the year.
Answers
An impact of the specific heat of the water on the planet is that islands and coastal places have moderately pleasant climates. Therefore, option A is correct.
The specific heat of water is relatively high compared to other substances. This means that water requires a significant amount of heat energy to increase its temperature. As a result, water has a stabilizing effect on the climate of coastal and island regions.
The high specific heat of the water helps to moderate temperature changes, resulting in milder and more pleasant climates in these areas.
Learn more about specific heat, here:
https://brainly.com/question/31608647
#SPJ1
Which of the following are compounds?A. H₂O, 02, and CH4B. H₂0 and 02C. 02 and CH4D. H₂O and CH4, but not 02
Answers
Answer: D) H2O and CH4 but not O2
A chemical compound consists of atoms of two or more chemical elements.
A gas has a volume of 543 ml. at a temperature of 75.0°C. What volume will the gas occupy at 20.2°C?
Answers
Answer:
V₂ = 457.49 mL
Explanation:
Given that,
Initial volume, \(V_1=543\ mL\)
Initial temperature, \(T_1=75^{\circ} C=75+273=348\ K\)
Final temperature, \(T_2=20.2^{\circ} C=20.2+273=293.2\ K\)
We need to find the final volume of the gas. The relation between the volume and the temperature of the gas is given by :
\(\dfrac{V_1}{T_1}=\dfrac{V_2}{T_2}\)
Put the respected values,
\(V_2=\dfrac{V_1T_2}{T_1}\\\\V_2=\dfrac{543\times 293.2}{348}\\\\V_2=457.49\ mL\)
So, the final volume of the gas is equal to 457.49 mL.
Please help me
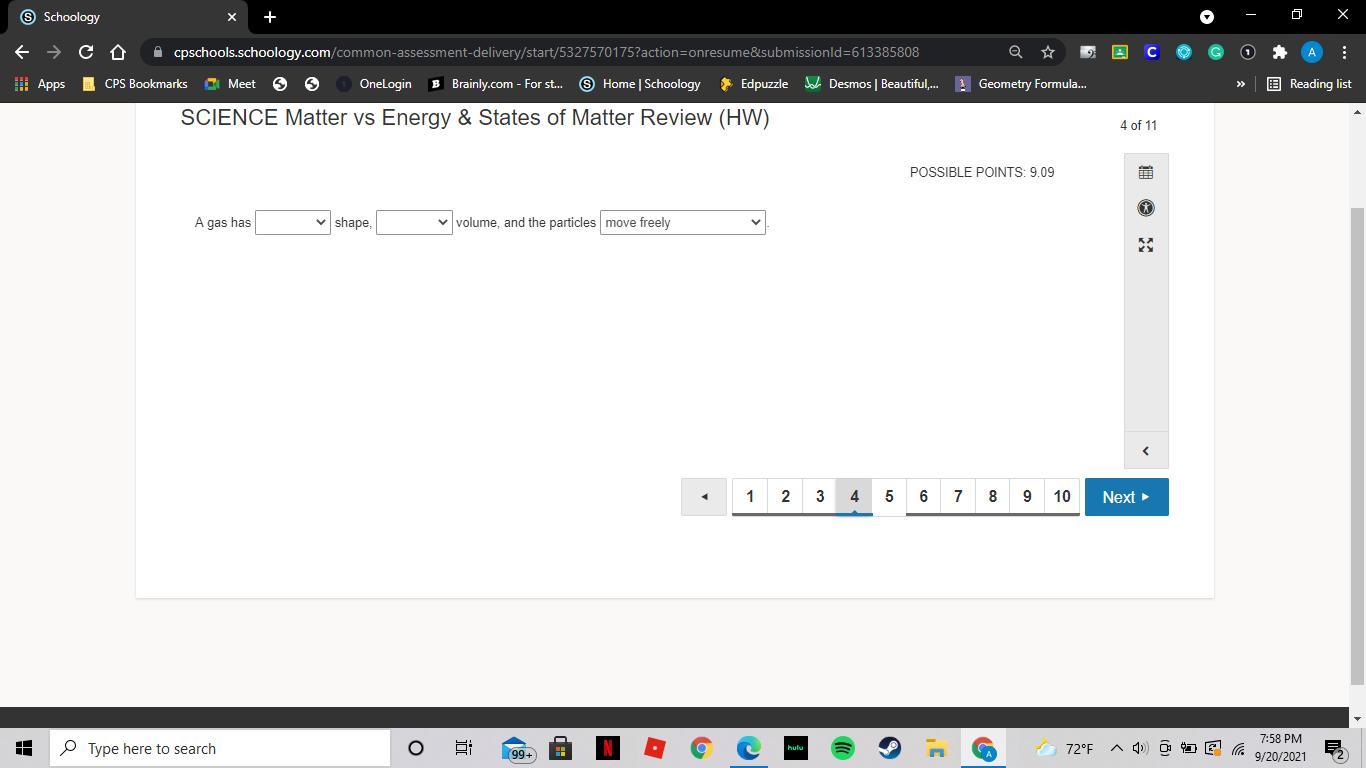
A gas has definite shape or indefinite shape
A gas has indefinite volume or definite volume?
Answers
A model of an atom shows eight electrons in rings that represent different energy levels. How many electrons are in each energy level?(1 point) Responses four in the first energy level, four in the second energy level four in the first energy level, four in the second energy level zero in the first energy level, eight in the second energy level zero in the first energy level, eight in the second energy level two in the first energy level, six in the second energy level two in the first energy level, six in the second energy level eight in the first energy level, zero in the second energy level eight in the first energy level, zero in the second energy level
Answers
The correct option is, two in the first energy level, six in the second energy level.
Define model of atom.
An atom is a fundamental unit of matter that cannot be disassembled chemically.
Depending on the energy of the electron, the orbital shells that an atom's electrons are arranged in are located at various levels within the structure of the atom. The initial energy level, or electron shell nearest to the atom's nucleus, is where electrons with the lowest energy can be found.
The following formula determines the maximum number of electrons that can be discovered at a specific energy level;
The number of electron = 2 × n²
Where;
n = The number of the different energy level
Therefore;
In the first energy when n = 1, the maximum number of electrons is provided as follows;
The number of electron = 2 × 1² = 2 electrons
In the second energy when n = 2, the maximum number of electrons is provided as follows;
The number of electron = 2 × 2² = 8 electrons
We have 2 electrons in the first energy level and the remaining 6 electrons in the second energy level because there are only 8 electrons in the atom.
To learn more about model of atom from the given link.
https://brainly.com/question/6258301
#SPJ1
9. Predict the full Robinson Annulation product (including all intermediates) and 10. Show the stepwise mechanism for the following reaction.

Answers
Can you help me with this I can’t fully get it
The other answer are
41%
50%
33%
Thank u

Answers
The percent yield of the reaction is 50 %.
What is the percent yield?We know that we have to use the balanced reaction equation so as to solve the problem that we have at hand here now.
We know that;
Number of moles of oxygen = 160 g/32 g/mol
= 5 moles
We now know that the liming reactant in this case is the oxygen. Thus we can be able to write here that;
Thus we know that the theoretical yield of the CO2 = 3 * 44 = 132 g
The percent yield = 66/132 * 100
= 50 %
This is the percent of the product that we can be able to obtain.
Learn more about percent yield:https://brainly.com/question/17042787
#SPJ1
So its not A so its B C or D plz help

Answers
Answer:
the answer is B
hope this helped!
The sun is ____star
Answers
The sun is a star.
If you were talking about that
the sun is a Star...............
I'm having difficult to draw the resonance structure of this molecule. Someone can help me, please

Answers
Antonio is a silversmith and works with various metals. At the end of the day, he reclaims and separates each metal by using an inexpensive toxic acid though other methods are available. The risk of fire as well as health dangers to Antonio is unknown. What should Antonio do
Answers
The question is incomplete, the complete question is;
Antonio is a silversmith and works with various metals. At the end of the day, he reclaims and separates each metal by using an inexpensive toxic acid though other methods are available. The risk of fire as well as health dangers to Antonio is unknown. What should Antonio do? A) Dispose of the used acid by pouring it down the drain since the government will take care of any environmental issues that come from polluting the water. B) Get a fire insurance policy and make sure to visit the doctor regularly. C) Switch to another potentially less dangerous method of separating each metal. D) Pour it out in back of the studio in the gutter.
Answer:
C) Switch to another potentially less dangerous method of separating each metal.
Explanation:
The importance of safety in manufacturing processes can not be over emphasized. Clearly, Antonio's method of separating each metal holds the potential of a fire outbreak or causing major health problem for Antonio.
The best thing that Antonio can do is to explore other less potentially dangerous methods of separating each metal since there are other available and possibly safer methods of achieving the same end.
Between thermal energy and heat
Answers
Thermal energy is heat it heats you up it give people heat it give away the heat it has until it is cold
Either you miswrote that or something else
2NO(g)+O2(g)→2NO2(g)
If 98.3L of NO2 forms, measured at 39.0∘C and 631 mmHg, what is the percent yield?
Answers
The percent yield of the reaction 2NO(g)+O₂(g)→2NO₂(g) is 7.98%.
Mole ratio of NO to NO₂ is 2:2 or 1:1. Therefore, the number of moles of NO₂ formed is equal to the number of moles of NO reacted.
For an ideal gas, PV = nRT
n = PV/RT where temperature is in kelvin
volume of NO₂ formed = 98.3 L
temperature = 39.0°C or 312.15 K
pressure = 631 mmHg or 0.830 atm
n(NO) = PV/RT \
= (0.830 atm)(98.3 L)/(0.0821 L·atm/mol·K)(312.15 K)
= 33.6 mol
Since, the ratio is 1:1 yield of NO₂ is also 33.6 mol.
Actual yield can be found by converting moles in molar mass of NO₂
NO₂: 1 × 14.01 + 2 × 16.00 = 46.01 g/mol
If the mass of NO2 obtained was 123.4 g. Then the number of moles of NO₂ is
n(NO₂) = m/M = 123.4 g/46.01 g/mol = 2.68 mol
% yield = (actual yield/theoretical yield) x 100%
= (2.68 mol/33.6 mol) x 100%
= 7.98%
Learn more about percent yield at:
brainly.com/question/2506978
#SPJ1
Can H2 be broken down? (Not H)
Answers
Hello, this is Bing. I can help you with your question. Based on the information I found on the web, **H2** can be broken down into its two atoms of hydrogen (H) by supplying enough energy to overcome the bond that holds them together⁴. This process is called **dissociation** and requires an energy equal to or greater than the **dissociation energy** of H2, which is about 436 kJ/mol⁴.
One way to break down H2 is by using **electricity** to split water (H2O) into hydrogen (H2) and oxygen (O2) through a process called **electrolysis**¹. In this process, water is decomposed into its elements by passing an electric current through it. The electric current is provided by a battery or another source of electricity and the water needs to have an **electrolyte**, such as salt or acid, added to it to make it conductive¹. Two electrodes, usually made of metal or other conductive material, are inserted into the water and connected to the battery. The electrode connected to the positive terminal of the battery is called the **anode** and the one connected to the negative terminal is called the **cathode**¹. When the electric current flows through the water, hydrogen gas bubbles form at the cathode and oxygen gas bubbles form at the anode¹. The overall chemical reaction for electrolysis of water is:
2 H2O → 2 H2 + O2
Another way to break down H2 is by using **heat** to cause a reaction between hydrogen and oxygen that produces water and releases a large amount of energy. This reaction is called **combustion** or **oxidation** and can be ignited by a spark or a flame³. The reaction is very fast and explosive and can be dangerous if not controlled. The overall chemical reaction for combustion of hydrogen is:
2 H2 + O2 → 2 H2O
I hope this helps you understand how H2 can be broken down and what methods are used to do so.
What is the primary function of the chromosome?
Answers
Answer:
Store the genetic instructions needed to specify traits.
In DNA, the primary function of chromosome is store the genetic instructions needed to specify traits.
What is DNA?
DNA is a hereditary material which is present in human beings as well as all other living organisms. Every cell which is present in an organism's body has DNA which is the same. Most of the DNA is situated in the cell's nucleus and small amount of it can be found in the cell's mitochondria as well.
Information which is stored in DNA is stored as codes made up of four chemical bases namely, adenine, thymine , cytosine and guanine.Human DNA consists of 3 billion bases .The order of the bases determines information which is required for building and maintaining an organism.
DNA bases are capable of pairing up with each other. Adenine pairs with thymine and guanine pairs up with cytosine .Each base is also attached to a sugar molecule and a phosphate group. A base, phosphate sugar are together called as nucleotides.
Learn more about DNA,here:
https://brainly.com/question/22499464
#SPJ6
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
For the following, identify the most likely value for x.a. BHx b. CHx c. NHx d. CH2Clx
Answers
Given :
a. \(BH_x\) b. \(CH_x\) c. \(NH_x\) d. \(CH_2Cl_x\) .
To Find :
Find the most likely vale of x for each one .
Solution :
a . \(BH_x\)
Because boron have valency of 3 .
So , x = 3 .
b . \(CH_x\)
Valency of carbon is 4 .
x = 4 .
c . \(NH_x\)
Valency of nitrogen is 3 .
Therefore , x = 3 .
d . \(CH_2Cl_x\)
Now ,we know valency of carbon is 4 and hydrogen is 1 .
Also , two hydrogen are already there .
So , only 2 electrons left to share .
Since , chlorine have valency of 1 .
Therefore , only 2 electrons of chlorine can connect .
x = 2 .
Hence , this is the required solution .
A piece of magnesium is in the shape of a cylinder with a height of 6.62 cm and a diameter of 2.34 cm. If the magnesium sample has a mass of 17.1 g, what is the density of the sample? (formula for volume of a cylinder: volume = r2h)
Answers
Answer:
0.600 g/cm³
Explanation:
Step 1: Given data
Height of the cylinder (h): 6.62 cmDiameter of the cylinder (d): 2.34 cmMass of the cylinder (m): 17.1 gStep 2: Calculate the volume of the cylinder
First, we have to determine the radius, which is half of the diameter.
r = d/2 = 2.34 cm/2 = 1.17 cm
Then, we use the formula for the volume of the cylinder.
V= π × r² × h
V= π × (1.17 cm)² × 6.62 cm
V = 28.5 cm³
Step 3: Calculate the density (ρ) of the sample
The density is equal to the mass divided by the volume.
ρ = m/V
ρ = 17.1 g/28.5 cm³
ρ = 0.600 g/cm³
For each question, determine the type of reaction, then balance the reaction.
Problems 1-4: 2 points each
1.
Al +
H, Te →
Al, Tez +
H
a. Type:
2.
_C12H26 +
O2 →
H2O +
CO2
a. Type:
3.
Au +
CuSO4 →
Cu +
AuSO4
a. Type:
4.
H₂O →
H2 +
a. Type:

Answers
Answer:
i know the answer but i dont have time bro
Why must the pH values of the mouth, stomach, and small intestine be different?
Answers
Answer:
so you don't digest your tounge
The isotope calcium-41 decays into potassium-41, with half-life of 1.03 x 105 years. There is a sample of calcium-41 containing 5 x 10° atoms. How
many atoms of calcium-41 and potassium-41 will there be after 4.12 › 105 years?
A.
3.125 x 108 atoms of calcium-41 and 4.375 x 10° atoms of potassium-41
6.25 x 108 atoms of calcium-41 and 4.6875 x 10° atoms of potassium-41
•
6.25 x 108 atoms of calcium-41 and 4.375 10° atoms of potassium-41
O D. 3.125 x 108 atoms of calcium-41 and 4.6875 › 10° atoms of potassium-41
Answers
3.125 × 10^8 atoms of calcium-41 and 4.6875 × 10^9 atoms of potassium-41.
Option D is correct
Isotope 41 of calcium is it?A rare and long-lived radioactive isotope of calcium is calcium-41 (41Ca).
# Given-
Half life = 1.03×10^5 years
- After 1.03×10^5 years (1 half life)
calcium-41 will be 50%
potassium-41 will be 50%
- After 2.06×10^5 years (2 half lives)
calcium-41 will be 25%
potassium-41 will be 75%
- After 3.09×10^5 years (3 half lives)
calcium-41 will be 12.5%
potassium-41 will be 87.5%
- After 4.12×10^5 years (4 half lives)
calcium-41 will be 6.25%
potassium-41 will be 93.75%
After 4.12×10^5 years,
Calcium-41 = 6.25/100 × 5 × 10^9 = 3.12×10^8 atoms
Potassium-41 = 93.75/100 × 5 × 10^9 = 4.69×10^9 atoms
To know more about Isotope 41 visit:-
https://brainly.com/question/29427598
#SPJ1
How many formula units are in 50.0g of Pb02?
Answers
There are approximately \(1.258 x 10^2^3\) formula units in 50.0 g of PbO2.
To solve this problem
We must utilize the molar mass of PbO2 (lead dioxide) and the idea of Avogadro's number to calculate the number of formula units in a given mass of PbO2.
The molar mass of PbO2 is calculated as follows:
1 atom of Pb (lead) has a molar mass of approximately 207.2 g/mol.
2 atoms of O (oxygen) have a combined molar mass of approximately 32.0 g/mol (16.0 g/mol per oxygen atom).
Therefore, the molar mass of PbO2 is:
Molar mass of PbO2 = (1 * molar mass of Pb) + (2 * molar mass of O)
= (1 * 207.2 g/mol) + (2 * 16.0 g/mol)
= 207.2 g/mol + 32.0 g/mol
= 239.2 g/mol
Now, we can use the molar mass to determine the number of formula units in 50.0 g of PbO2.
Number of moles = Mass (in grams) / Molar mass
= 50.0 g / 239.2 g/mol
≈ 0.209 moles (rounded to three decimal places)
Since 1 mole of any substance contains Avogadro's number of particles \((approximately 6.022 x 10^2^3),\)we can calculate the number of formula units by multiplying the number of moles by Avogadro's number:
Number of formula units = Number of moles * Avogadro's number
\(= 0.209 moles * (6.022 x 10^2^3 formula units/mole)\)
≈\(1.258 x 10^2^3 formula units\)
Therefore, there are approximately\(1.258 x 10^2^3\) formula units in 50.0 g of PbO2.
Learn more about molar mass here : brainly.com/question/21334167
#SPJ1
Data Table 3: Polystyrene Test Tube, 12x75mm
Volume of water at room temperature (V1 in ml)
Volume of gas in polystyrene tube at boil (V2 in mL)
Temperature of gas at boil inside polystyrene tube (°C)
Volume of gas in polystyrenetube at room temperature (V3 in mL)
Temperature of gas at room temperature (°C)
Need help with data table 3: polystyrene test tube, 12x75mm
Answers
Answer:
Experiment 8 E Data Table 3 fl Data Table 4 fl Data Table 5 fl Data Table 6 Data Table 3: Polystyrene Test Tube, 12x75mm Volume of water at room temperature (V1 in mL) Volume of gas in polystyrene tube at boil (V2 in mL) Temperature of gas at boil inside polystyrene tube (°C) Volume of gas in polystyrenetube at room temperature (V3 in mL) Temperature of gas.
Explanation:
Hope this helps
Mark as Brainliest please
What would the hydroxide ion concentration be if the hydrogen ion concentration was 1 x 10-3 M?
Answers
Answer:
1 x 10⁻¹¹ M
Explanation:
(Step 1)
Determine the pH.
pH = -log[H⁺]
pH = -log[1 x 10⁻³ M]
pH = 3
(Step 2)
Determine the pOH.
pH + pOH = 14
3 + pOH = 14
pOH = 11
(Step 3)
Determine the hydroxide (OH⁻) concentration.
[OH⁻] = 10^-pOH
[OH⁻] = 10⁻¹¹
[OH⁻] = 1 x 10⁻¹¹ M
Using the van der waals equation, the pressure in a 22.4 L vessel containing 1.50 mol of chlorine gas at 0.00 c is____________atm. (a= 6.49L^2-atm/mol^2, b=0.0562 L/mol)
A. 1.50
b. 0.676
c. 0.993
d. 1.48
e. 1.91
Answers
Answer:
D. 1.48atm
Explanation:
Van der waals equation is given as:
(P +an²/v²) (v - nb) = nRT
Where;
P = pressure (atm)
V = volume (L)
R = gas constant (0.0821 Latm/molK)
a and b = gas constant specific to each gas
T = temperature (K)
n = number of moles
According to the given information; V = 22.4L, T = 0.00°C (273.15K), R = 0.0821 Latm/molK, a = 6.49L^2-atm/mol^2, b = 0.0562 L/mol, n = 1.5mol
Hence;
(P + 6.49 × 1.5²/22.4²) (22.4 - 1.5×0.0562) = 1.5 × 0.0821 × 273.15
(P + 6.49 × 2.25/501.76) (22.4 - 0.0843) = 33.638
(P + 0.0291) (22.316) = 33.638
22.316P + 0.649 = 33.638
22.316P = 33.638 - 0.649
22.316P = 32.989
P = 32.989/22.316
P = 1.478
P = 1.48atm
From the given electron configurations, predict which one is for a representative element?
A) 1s22s22p63s23p64s2
B) 1s22s22p63s23p63d74s2
C) 1s22s22p63s23p63d64s2
D) 1s22s22p63s23d5
Answers
From the given electron configurations, predict which one is for a representative element is 1s₂, 2s₂, 2p₆,3s₂, 3p₆, 3d₆, 4s₂.
What is element ?A chemical element is a species of atoms, including the pure material made entirely of that species, that have a specific number of protons in their nuclei. Chemical elements, unlike chemical compounds, cannot be reduced by any chemical process into simpler molecules.
The most basic form of a material is an element. In general, it cannot be streamlined or divided into smaller parts. A component of an element is an atom. A certain element only has one kind of atom per atom. Protons, neutrons, and electrons, which are subatomic particles, make up atoms.
We begin by applying the aufbau principle to the prediction of the electron configuration of an atom. It instructs us to fill the lowest energy accessible orbital, one electron at a time. It operates through element 18 (argon) in a filling pattern that is simple to predict: 1s, 2s, 2p, 3s, then 3p.
Thus, option C is correct.
To learn more about element, follow the link;
https://brainly.com/question/13025901
#SPJ6
HELP ME FAST
How many moles are in 325 g of MgCO3?
0.161 moles
0.269 moles
1.98 moles
3.85 moles
Answers
Answer:
3.85
Explanation:
Calculate molar mass, starting with each element, then find grams per mole, then convert.
Which symbol represents salt?
Question 10 options:
C2H2
O2
C6H12O6
KBr
Answers
Answer:none
Explanation:none of these represent salt,i know that it's NaCl