How many years are equal to 1,500,000 seconds?
Answers
Answer:
2.853881279 years
Explanation:
I multiplied 365 times 24 to get 8760 hours in a year. I then multiplied this by 60 to get 525600 seconds per year. Lastly, I divided 1500000 by 525600 in order to get the answer. Hope this helps!
Related Questions
Reforestation projects, where only one species is planted in an area, result in..
1. the likelihood of an unsustainable ecosystems.
2. limited biodiversity.
3. a population at increased risk of disease or insect invasion.
4. all of the above
Multiple choice
Answers
Several atmospheric gases contribute to global warming: carbon dioxide, methane, and nitrous oxide. Considering this, which choice would do the LEAST to directly reduce atmospheric carbon dioxide.
Answers
Answer:
A
help u and it's A
Explanation:
an aqueous potassium carbonate solution is made by dissolving 6.63 moles of k2co3 in sufficient water so that the final volume of the solution is 2.80 l . calculate the molarity of the k2co3 solution.
Answers
The molarity of the K₂CO₃ solution is 2.36 M.
MolarityThe unit of molarity is molar or M is the same as moles per liter, when n moles of a compound appear that have been dissolved in V liters of solution, the molarity formula is \(M = n/V\)
M = molarity
n = number of moles
V = volume of solution
The concentration of the solution states the quantitative composition of the substance that has been dissolved and the solvent in the solution. Determination of the concentration or vice versa depends on the number of solution ratios of each substance that enters. Chemical reactions occur during the process and result from the presence of the same solution.
So, the molarity of the K₂CO₃ solution is:
\(M =6.63/2.8\)
\(=2.36\) M
Learn more about molarity here: https://brainly.com/question/24305514#SPJ4
Reasons why recycling scrap copper is more sustainable than extracting copper from copper ores
Answers
Answer:
Recycling scrap copper is more sustainable than extracting copper from copper ores due to the following reasons:
Reduces energy consumption: Recycling scrap copper requires less energy compared to mining, extracting, and processing copper ores. This results in a reduction in greenhouse gas emissions and a decrease in energy consumption.
Conservation of natural resources: Recycling scrap copper conserves natural resources because it reduces the need for mining copper ores. Mining copper ores often leads to the destruction of ecosystems, soil erosion, and the emission of harmful pollutants into the environment.
Reduction in landfill waste: Recycling scrap copper helps to reduce the amount of waste in landfills. Copper is a valuable resource, and recycling it ensures that it is reused instead of ending up in landfills.
Cost-effective: Recycling scrap copper is often cheaper than extracting copper from copper ores. This is because recycling eliminates the cost of mining, extracting, and processing copper ores.
Preservation of water resources: Extracting copper from copper ores requires large amounts of water, which can lead to water shortages in some regions. Recycling scrap copper requires less water, thus helping to preserve water resources.
Overall, recycling scrap copper is more sustainable than extracting copper from copper ores because it reduces energy consumption, conserves natural resources, reduces landfill waste, is cost-effective, and helps to preserve water resources.
Explanation:
Bases holding two single strands of DNA together into a double strand of DNA interact through ___________ bonds. hydrogen covalent chemical carbon ionic
Answers
Option (A) is correct. Bases holding two single strands of DNA together into a double strand of DNA interact through Hydrogen bonds.
The bases holding two single strand of the DNA molecule is held together by hydrogen bonding. These bonding occurs between the nitrogenous bases in the two strands of DNA. The specific base pairings for DNA molecules are the Adenine-Thymine and the Cytosine-Guanine. The DNA double helix is held together by two types of bonds that is covalent bond and hydrogen bond. Covalent bonds occur within each linear strand and strongly bond the bases, sugars, and phosphate groups that is both within each component and between components. Hydrogen bonds occur between the two strands and involve a base from one strand with a base from the second in complementary pairing in the DNA. These hydrogen bonds in the DNA are individually weak but collectively quite strong.
To learn more about Hydrogen bonding
https://brainly.com/question/1426421
#SPJ4
The complete question is,
Bases holding two single strands of DNA together into a double strand of DNA interact through ___________ bonds.
A. hydrogen
B. covalent
C. chemical carbon
D. ionic
3
3) Write any 4 important of
4 important of measurement
Science

Answers
Answer:
A measurement is the action of measuring something, or some amount of stuff. So it is important to measure certain things right, distance, time, and accuracy are all great things to measure. Measurements can also allow us to make desicions based on the outcome of the measurement
A piece of copper wire is 180 cm long. how long is the wire in millimeters? how many
40 mm segments of wire can be cut from the length
Answers
Centimeters and millimeters are both interconvertible units to measure length. The conversion factor is as follows:
1 centimetre = 10 millimetres
This means that if a copper wire is 180cm long, the wire would be;
180 × 10 = 1800mm long
Also, the number of 40 mm segments of wire that can be cut from the length is calculated as follows:
1800mm/40mm = 45
Learn more about conversion at: https://brainly.com/question/12103054
#SPJ1
What aspect of a hurricane causes the most damage and why?
Answers
Answer:
Hurricanes can be broken down into four quadrants and while all sides are dangerous, the most destructive is the right front quadrant. This is due to the forward motion contributing to the rotation of the storm. This side of the storm tends to have higher winds, higher storm surge, seas, and the highest rainfall.
What forces typically hold ions together?
O A. Intermolecular forces
OB. Ionic attractions
OC. Metallic bonds
O D. Covalent bonds
Answers
Answer: Ionic attractions
Explanation:
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.
Mercury melts at -39°C and boils at 356.9°C. What is its state at -20°C?
Answers
Answer:
At -20°C the mercury state will be liquid
Explanation:
The melting point of a substance is the temperature at which it changes from solid state to a liquid state, and the boiling point is the temperature at which it changes from liquid state to a gaseous state.
If mercury melts at -39°C and boils at 356.9°C, it means that if the temperature is lower than -39°C, mercury will remain al solid state. Between -39°C and 356.9°C, the state of mercury will be liquid and, above 356.9°C mercury will remain at gaseous state.
So, at -20°C the mercury state will be liquid, because that temperature it is between -39°C and 356.9°C.
i need help again guyss

Answers
a 10 ml sample of 0.20 m hbr solution is titrated with 0.10 m naoh. what volume of naoh is required to reach the equialvence point?
Answers
The volume Naoh is required to reach the equivalent point is L.
Equivalent point is the point in the chemical reaction when there is acid or base to neutralize the solution. It is also known as stoichiometric point. The moles of the titrant is equal to the moles of the unknown concentration in the titration.
The volume of the nbr solution is 0.20m and the concentration of nbr is 10ml. and concentration of the Noah is 0.10.so, here C1 is 0.20m and v1 is 0.010l and C2 is 0.10m .
so putting all the value sin the expression,
C1 V1 = C2 V2
where C1 is the concentration of the acid ,c2 is the concentration of the base,V1 is the volume of the acid and V2 is the volume of the base.
V2=C1. V1 / C2
= 0.20 .0.010 /0.10
= L of Naoh.
To learn more about Equivalent point in titration please visit:
https://brainly.com/question/2496608
#SPJ4
ssume it takes 5.00 min to fill a 45.0−gal gasoline tank. (1 U.S. gal=231 in.
3
) ta) Calculate the rate at which the tank is filled in gallons per second. gal/5 (b) Calculate the rate at which the tank is filled in cubic meters per second. m
3
/5 (c) Determine the time interval, in hours, required to fill a 1.00−m
3
volume at the same rate. (1 U.S. gal =231 in.
3
)
Answers
(a) The rate at which the tank is filled is 9 gallons per minute or 1.5 gallons per second.
(b) The rate at which the tank is filled is approximately 0.0571 cubic meters per second.
(c) It would take approximately 6.28 hours to fill a 1.00 cubic meter volume at the same rate.
To calculate the rate at which the tank is filled in gallons per second, we divide the volume of the tank (45.0 gallons) by the time taken to fill it (5.00 minutes). This gives us a rate of 9 gallons per minute. To convert it to gallons per second, we divide by 60 since there are 60 seconds in a minute, resulting in 1.5 gallons per second.
To convert the rate of filling from gallons per second to cubic meters per second, we need to convert gallons to cubic meters. Since 1 U.S. gallon is equal to 231 cubic inches and 1 cubic meter is equal to 1,000,000 cubic centimeters, we can use unit conversions to find that approximately 0.0571 cubic meters are filled per second.
To determine the time interval required to fill a 1.00 cubic meter volume at the same rate, we can use the rate calculated in part (b). Dividing the volume of 1.00 cubic meter by the rate of 0.0571 cubic meters per second, we find that it would take approximately 17.5 seconds to fill 1.00 cubic meter. Converting this to hours, we divide by 3600 (the number of seconds in an hour), which gives us approximately 6.28 hours.
Learn more about gallons
brainly.com/question/31702678
#SPJ11
What is a solution that is able to dissolve additional solute?
Answers
Unsaturated solution is a solution in which more of the solute can be dissolved at a given temperature.
What is unsaturated solution with example?
Unsaturated solutions are any in which the concentration of the solute is less than the solute's saturation point. Two tablespoons of salt dissolved in one litre of water serves as an illustration. Acetic acid is the solute and water is the solvent in vinegar, which is an unsaturated solution.
Hence Unsaturated solution is a correct answer.
To know more about Unsaturated solution follow link
https://brainly.com/question/18997806
#SPJ4
a 1.25-molar solution of a weak monoprotic acid is 9.2% ionized. calculate the ph of the solution.
Answers
The pH of the solution is approximately 0.939 , we can start by determining the degree of ionization (α) of the weak acid, which is given as 9.2%. The degree of ionization represents the fraction of the acid that has dissociated into ions.
We know that the initial concentration of the weak acid is 1.25 M. Let's denote the initial concentration of the acid as [HA] and the concentration of the dissociated H+ ions as [H+]. The concentration of undissociated HA molecules can be represented as [(1 - α) * [HA]].
Given that α = 9.2% = 0.092, we can write the equation for the concentration of H+ ions:
[H+] = α * [HA]
Substituting the values:
[H+] = 0.092 * 1.25 M
[H+] ≈ 0.115 M
Now that we have the concentration of H+ ions, we can calculate the pH using the formula:
pH = -log[H+]
pH = -log(0.115)
pH ≈ 0.939
Therefore, the pH of the solution is approximately 0.939.
To learn more about pH refer here:
https://brainly.com/question/2288405#
#SPJ11
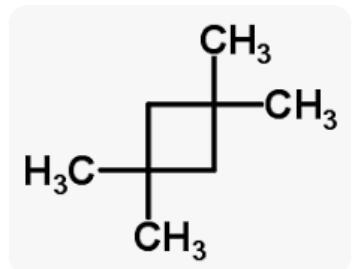
1-methylcyclobutane how many monochloro substitution products are produced when the alkane below is chlorinated? consider both constitutional isomers and stereoisomers. the number of monochloro substitution products is
Answers
The number of mono-chloro substitution isomers are 5 when the alkane below is chlorinated.
Even when several chlorination are conceivable, monochlorination, as its name suggests, refers to chlorination with a single atom. By swapping out one of an alkane's hydrogen atoms for a chlorine atom, the alkane can be monochlorinated. When UV radiation is present, this is achieved by adding chlorine to the alkane. These conditions lead to the splitting of the chlorine molecule into chlorine free radicals. As a result, when propane is chlorinated, 1-chloropropane and 2-chloropropane are created as mono-chlorinated molecules. A hydrogen atom interacts with a free radical during an interaction with an alkane to create a primary methyl radical. Since all hydrogen atoms in light alkanes are identical, they all have the same chance of being replaced.
To learn more about isomers click here https://brainly.com/question/12796779
#SPJ4
Question III A+ 2B is elementary reversible gas phase reaction that is conducted at 540 °F and 3 atm in a PFR. The feed rate is 75 lb mol/h with 40% A and 60% inert material in the feed. The specific reaction rate k = 1.6 s and the concentration equilibrium constant K = 0.0055 lb mol/ft³. Calculate volume of reactor and space-time if 75 % equilibrium conversion is achieved.
Answers
To calculate the volume of the reactor and space-time for a reversible gas phase reaction, A+2B, conducted at 540 °F and 3 atm with a feed rate of 75 lb mol/h and 40% A, and an equilibrium conversion of 75%, we need to consider the specific reaction rate and the concentration equilibrium constant.
The space-time for a reactor is defined as the volume of the reactor divided by the feed rate. To calculate the volume of the reactor, we first determine the molar flow rate of component A, which is 75 lb mol/h * 0.40 = 30 lb mol/h. Then, we divide the molar flow rate of A by the specific reaction rate to obtain the volume: Volume = 30 lb mol/h / (1.6 s * 3600 s/h) = 5.2083 ft³.
To calculate the space-time, we divide the volume by the feed rate: Space-time = 5.2083 ft³ / 75 lb mol/h = 0.0694 ft³/lb mol/h.
Therefore, the volume of the reactor is 5.2083 ft³ and the space-time is 0.0694 ft³/lb mol/h.
Learn more about equilibrium constant here: brainly.com/question/29802105
#SPJ11
*URGENT ILL RATE YOU BRAINLIEST ASWELL* What is the volume of a rectangular prism with a length of 10cm, width of 2cm and a height of 8 cm?
Remember: V = length x width x height
Answers
Answer:
160 cm
Explanation:
since you said that v=l x w x h
then you will only have to multiply all of them, 10cm x 2 cm x 8cm, and the answer is 160 cm
A compound contains 64.3% carbon, 7.14% Hydrogen, and 28.6% Oxygen. What is the molecular formula for this substance?
Answers
C₃H₄O. is the molecular formula for this substance.
What is an empirical formula and how is it calculated?An empirical formula is a compound's chemical formula that only specifies the ratios of the elements it contains and not the precise number or arrangement of atoms. This would be the compound's element with the lowest whole number ratio.Given :
Percentage of hydrogen = 7.14%
Percentage of carbon = 64.3%
Percentage of oxygen = 28.6%
To find Empirical formula
Number of gram atoms of H = 7.14 / 1.01 = 7.1
Number of gram atoms of O = 28.6 / 16 = 1.8
Number of gram atoms of C = 64.3 / 12 = 5.4
Atomic ratio:
C : H : O
5.4/1.8 : 7.1/1.8 : 1.8/1.8
3 : 4 : 1
C : H : O = 3 : 4 : 1
Empirical formula is C₃H₄O.
To learn more about empirical formula refer,
https://brainly.com/question/20806970
#SPJ1
About ______ impact the creators have been recognized on earth.
A) one thousand
B) 50
C)500
D)150
Answers
What factors generally determine whether a reaction happens or not?
A. Reaction rate and color
B. Presence of water and salt
C. Enthalpy and entropy
D. Keg and Ka
Answers
Answer:
Explanation:
The answer is C Enthaply and entropy because temperature plays a big role in reactions
Answer:
c
Explanation:
a p e x :)
for each of the following
i) write a skeleton equation
ii) write a correct balanced chemical equation
iii) classify reaction by type
1) calcium metal and water react, giving hydrogen gas and calcium hydroxide
2) aluminum metal quickly reacts with the oxygen in the air to produce aluminum oxide
3) hydrogen sulphate (sulphutic acid) and sodium hydroxide react, producing sodium sulphate and water.
4) sodium chloride and oxygen are produced by heating sodium chlorate.
5) silver nitrate and potassium phosphate react together producing silver phosphate and potassium nitrate.
6) aluminum oxide and copper metal are the products of a reaction between copper (II) oxide and aluminum metal.
7)magnesium and phosphorus (P4) react together, producing magnesium phosphide.
8) lead (II) nitrate and potassium iodide react producing lead (II) iodide a bright yellow precipitate and potassium nitrate which stays in solution.
Answers
Answer:
1) Calcium metal and water react, giving hydrogen gas and calcium hydroxide
• Ca(s) + H2O Ca(OH)2 (aq) + H2(g)
• Ca(s) + 2H2O Ca(OH)2 (aq) + H2(g)
• Exothermic combination reaction
2) aluminum metal quickly reacts with the oxygen in the air to produce aluminum oxide
• Al + 02 = Al2O3
• 4 Al + 302 = 2Al2O3
• Combination reaction
3) Hydrogen sulphate (sulphutic acid) and sodium hydroxide react, producing sodium sulphate and water.
• NaOH + H2SO4 = Na2SO4 + 2H2O
• 2NaOH + H2SO4 = Na2SO4 + 2H2O
• Double Displacement reaction
4) sodium chloride and oxygen are produced by heating sodium chlorate.
• NaclO3 = NaCl + O
• 2NaclO3 = 2NaCl + 3 O
• Decomposition reaction
6) aluminum oxide and copper metal are the products of a reaction between copper (II) oxide and aluminum metal.
• Al + CuO = Al2O3 + Cu
• 2 Al + 3CuO = Al2O3 + 3 Cu
• Oxidation and Reduction
8) lead (II) nitrate and potassium iodide react producing lead (II) iodide a bright yellow precipitate and potassium nitrate which stays in solution.
• Pb(No3)2 +KI = PbI2 + KNO3
• Pb(No3)2 +2KI = PbI2 + 2KNO3
• Double displacement reaction
Explanation:
Answer:
1. Skeleton: Ca + H₂O ⇒ H₂ + Ca(OH)₂
Balanced: Ca + 2H₂O ⇒ H₂ + Ca(OH)₂
Type: Single displacement / single replacement
2. Skeleton: Al + O₂ ⇒ Al₂O₃
Balanced: 4Al + 3O₂ ⇒ 2Al₂O₃
Type: Synthesis
3. Skeleton: H₂SO₄ + NaOH ⇒ Na₂SO₄ + H₂O
Balanced: H₂SO₄ + 2NaOH ⇒ Na₂SO₄ + 2H₂O
Type: Double displacement / double replacement
4. Skeleton: NaClO₃ ⇒ NaCl + O₂
Balanced: 2NaClO₃ ⇒ 2NaCl + 3O₂
Type: Decomposition
5. Skeleton: AgNO₃ + K₃PO₄ ⇒ Ag₃PO₄ + KNO₃
Balanced: 3AgNO₃ + K₃PO₄ ⇒ Ag₃PO₄ + 3KNO₃
Type: Double displacement / double replacement
6. Skeleton: Cu(OH)₂ + Al ⇒ Al(OH)₃ + Cu
Balanced: 3Cu(OH)₂ + 2Al ⇒ 2Al(OH)₃ + 3Cu
Type: Single replacement / single displacement
7. Skeleton: Mg + P₄ ⇒ Mg₃P₂
Balanced: 6Mg + P₄ ⇒ 2Mg₃P₂
Type: Synthesis
8. Skeleton: KNO₃ + PbI₂ ⇒ KI + Pb(NO₃)₂
Balanced: 2KNO₃ + PbI₂ ⇒ 2KI + Pb(NO₃)₂
pls ans quicckk two q
Answers
Answer:
there is nothing here
Explanation:
What is the difference between high spin and low spin configurations in tetrahedral coordination complexes?
Answers
In tetrahedral coordination complexes, the difference between high spin and low spin configurations lies in the arrangement of electrons in the d orbitals of the central metal ion.
In high spin complexes, the electrons are distributed across the d orbitals in a way that maximizes their pairing, leading to a higher number of unpaired electrons and a weaker ligand field. As a result, these complexes tend to have higher magnetic moments and weaker bond strengths. In contrast, in low spin complexes, the electrons are arranged in a way that minimizes their pairing, leading to a lower number of unpaired electrons and a stronger ligand field. These complexes tend to have lower magnetic moments and stronger bond strengths. The choice of high spin or low spin configuration depends on factors such as the nature of the ligands and the oxidation state of the metal ion.
More on tetrahedral coordination: https://brainly.com/question/29805754
#SPJ11
a protein contains four disulfide bonds. in order to break these bonds nad vs nadhresearchers added a minimum of:
Answers
A protein contains four disulfide bonds. In order to break these bonds researchers added a minimum of 4 moles of NADH for each mole of protein.
Proteins mature in the cell, forming protein disulfide bonds between the sulfur atoms of two cysteine amino acids (the cystine residue). These connections have grown over time as eukaryotic proteins have evolved, and once obtained, they have nearly always been kept.
The oxidized form of NAD+ is one in which it has lost an electron. As the molecule's reduced form, NADH receives the electron that was lost by NAD+. Disulfide bond is reduced by NADH.
NAD, also known as nicotinamide adenine dinucleotide, is essential for a variety of cellular processes. The process by which NAD is changed from its oxidized form (NAD+) to its reduced form (NADH) and back again gives the cell a way to absorb and give electrons.
To know more about NADH
https://brainly.com/question/24976306
#SPJ4
Perform the following
mathematical operation, and
report the answer to the correct number of significant figures.
7.68 x 4.564 = [?]
Answers
Above which temperature does the thermal conductivity of water start to fall?.
Answers
Answer:
melting point (550 K) up to 650 K.
Explanation:
The thermal conductivity exhibits a sharp drop in the temperature interval from the melting point (550 K) up to 650 K. At higher temperatures the thermal conductivity exhibits almost no temperature dependence.
What are the 4 ideas in Dalton's atomic model?
Answers
Dalton's atomic model, proposed by John Dalton in 1803, was one of the first scientific explanations of the nature of atoms. It is based on four key ideas: Atoms are indivisible and indestructible; Atoms of different elements have different atomic weights; Atoms of the same element are identical; Atoms combine in definite ratios to form compounds.
Atoms are indivisible and indestructible. This means that atoms cannot be broken down into smaller particles, and they cannot be created or destroyed.
Atoms of different elements have different atomic weights. This means that atoms of one element are different from atoms of another element based on their weight.
Atoms of the same element are identical. This means that all atoms of a given element have the same properties and characteristics.
Atoms combine in definite ratios to form compounds. This means that when atoms of different elements combine to form a compound, they do so in a specific ratio.
To know more about Dalton's atomic model here:
https://brainly.com/question/14364728#
#SPJ11
Pls solve this science thing free brainlest :)

Answers
it's the third pic
