How many kingdoms are in the domain Eukarya?
one
four
six
nine
Answers
Answer:
Four kingdoms:
The kingdoms in the domain Eukarya are Protista, Fungi, Plantae, and Animalia.
Answer: Four Kingdoms! I hope you get this question!
Explanation:
Related Questions
List all possible values of the angular momentum quantum number l for an electron in the L(n=2) shell of an atom.
Answers
In quantum mechanics, the angular momentum quantum number "l" defines the shape of the atomic orbital. The l value is an integer ranging from 0 to (n-1) where n is the principal quantum number.
Therefore, for an electron in the L(n=2) shell of an atom, the possible values of the angular momentum quantum number l would range from 0 to 1, since n=2.
This is because the L shell is the second shell, which has n=2. Therefore, it can have subshells with l=0 and l=1, also known as the s and p subshells respectively.
The angular momentum quantum number also has an effect on the energy of the electron, with higher l values having higher energy.
Thus, the possible values of the angular momentum quantum number l for an electron in the L(n=2) shell of an atom are l=0 and l=1.
To know more about quantum mechanics refer here: https://brainly.com/question/23780112#
#SPJ11
Which compound is a hydrocarbon?
C 2 H 6
H 2 O
CO 2
C 6 H 12 O 6
Answers
Answer:
C2H6 OR C6H12O6 THE ANSWER
Explanation:
C2H6
!!!!!!!!!!
because it has both hydrogen and carbon as given in the word "Hydro-carbon"
What is the mass of 5.119 102 molecules of copper sulfate
(CuSO4)?
Answers
Answer:
Mass = 135.66 ×10⁻²¹ g
Explanation:
Given data:
Number of molecules of CuSO₄= 5.119×10²
Mass of CuSO₄= ?
Solution:
The given problem will solve by using Avogadro number.
1 mole contain 6.022×10²³ molecules
5.119×10² molecules ×1 mol / 6.022×10²³ molecules
0.85×10⁻²¹ mol
Mass in grams:
Mass = number of moles × molar mass
Mass = 0.85×10⁻²¹ mol × 159.6 g/mol
Mass = 135.66 ×10⁻²¹ g
A gaseous mixture containing 1.5 mol of Ar and 3.5 mol of CO2 has a total pressure of 7.0 atm . What is the partial pressure of CO2.
Answers
Answer:
4.9 atm
Explanation:
Given:
Total pressure = 7.0 atm
(Total number of moles)=[ (1.5 mol arg) + (3.5 mol co2) ]
= 5 moles
Let Partial pressure of CO2 gas = P(CO2)
✓✓partial pressure of CO2 can be calculated using below expresion.
P(CO2) / Total pressure = (moles of CO2 gas) / Total number of moles
✓✓ Let us substitute the values into the above expresion.
[P(CO2) / 7.0 atm ] = [3.5 mol / 5 moles]
P(CO2)= [7 atm × 3.5 moles] / [5 moles]
= 4.9 atm
Hence, the partial pressure of CO2 is 4.9 atm
Answer:
Partial pressure of CO₂ = 4.9 atm
Explanation:
From the question given above, the following data were obtained:
Mole of Ar = 1.5 moles
Mole of CO₂ = 3.5 moles
Total pressure (Pₜ) = 7.0 atm
Partial pressure of CO₂ =?
Next, we shall determine the mole fraction of CO2. This can be obtained as follow:
Mole of Ar = 1.5 moles
Mole of CO₂ = 3.5 moles
Total mole = 1.5 + 3.5
Total mole = 5 mole
Mole fraction of CO₂ = mole of CO₂ / total mole
Mole fraction of CO₂ = 3.5 / 5
Mole fraction of CO₂ = 0.7
Finally, we shall determine the partial pressure of CO₂. This can be obtained as follow:
Total pressure (Pₜ) = 7.0 atm
Mole fraction of CO₂ = 0.7
Partial pressure of CO₂ =?
Partial pressure of CO₂ = mole fraction of CO₂ × total pressure
Partial pressure of CO₂ = 0.7 × 7
Partial pressure of CO₂ = 4.9 atm
PLS HELP ASAP DUE AT 11:59 AND IM MARKING BRAINLIEST
PLS DO NOT ANSWER IF YOUR NOT SURE ABOUT YOUR ANSWER. this is science btw.

Answers
Answer:
In the followings below, the one that doesn't attract to magnets is:
Option 4, Aluminum.
Explanation:
Mainly because of the aluminum's crystal structure.
Hope this helped you out! ^^
#CarryOnLearning
Answer:
Aluminum
Explanation:
Ferromagnetic Metals
Ferromagnetic metals and alloys are the only magnetic metals. Out of all of the elements on the periodic table, there are only 3 metals that are attracted to magnets: iron, nickel, cobalt.
Aluminum
Aluminum is a nonferrous metal (meaning not including iron or steel) that does not include any ferromagnetic metals. Additionally, aluminum itself is not magnetic, so there is no attraction between aluminum and a magnet.
dose someone like to talk and will you help me to, if not we can talk

Answers
Answer:
wsppp
Explanation:
Answer:
hi.. (:
Explanation:
What was your hypothesis regarding the change in urine volume comparing subjects who drank water and subjects who drank sports drink? briefly state the physiologic basis for your prediction. did your results support your prediction? if not, provide a possible explanation.
Answers
My hypothesis regarding the change in urine volume was that subjects who drank water would have a higher urine volume compared to those who drank a sports drink. This is because water is a pure hydrating agent that does not contain any added sugars or electrolytes, unlike sports drinks.
Electrolytes in sports drinks cause the body to retain more water and decrease urine output, while added sugars increase urine output. Therefore, the physiologic basis for my prediction is that drinking water would lead to more frequent urination and higher urine volume due to the absence of any added electrolytes and sugars.
In terms of the results, they supported my prediction as the subjects who drank water had a significantly higher urine volume compared to those who drank a sports drink. However, there were a few subjects who had unexpected results where they had a higher urine volume after drinking a sports drink. This could be due to individual variations in their physiology, hydration status, or even their fluid intake before the study. Therefore, it is important to take into account individual variations when interpreting the results.
Learn more about urine volume at brainly.com/question/27454851
#SPJ11
Answer:
urine
Explanation:
urine tastes good :oP
Convert 1.25 ug into hg
Answers
The answer is 1.25e-8.
When an atom loses an electron, it becomes d. ionized. e. a plasma. a. dissociated. b. an isotope. c. sublimated.
Answers
Answer:
When an atom loses an electron it becomes ionized
true/false : _ is a term for underground conductors between the utility electric supply system and the service point
Answers
False. The term for underground conductors between the utility electric supply system and the service point is not specified in the statement.
However, commonly, these underground conductors are referred to as service cables or service conductors. They are responsible for delivering electricity from the utility electric supply system to the service point, which is typically the location where the electrical service is connected to the customer's premises. The specific term may vary depending on the region or electrical system, but "_," as presented in the statement, does not accurately describe this type of underground conductor.
To learn more about conductor click here:brainly.com/question/14405035
#SPJ11
Step 1: Gather materials and necessary information. a) You will collect the following information about your element: • Periodic table information including type of element, atomic number, atomic mass, number of protons, number of electrons, number of neutrons, period number, group number, and group name • Physical and chemical properties of the element • When, where, and by whom the element was discovered • Where the element is found and how it is obtained • Uses for the element and/or products made from the element • Images of the element b) Be sure to keep a list of your references so you can cite them later. c) Ask your teacher where you should save your presentation as you work on it. Your teacher may also have specific guidelines about the file name you should use.
Answers
Answer:
We hold these truths to be self-evident, that all men are created equal, that they are endowed by their Creator with certain unalienable Rights, that among these are Life, Liberty and the pursuit of Happiness.--That to secure these rights, Governments are instituted among Men, deriving their just powers from the consent of the governed, --That whenever any Form of Government becomes destructive of these ends, it is the Right of the People to alter or to abolish it, and to institute new Government, laying its foundation on such principles and organizing its powers in such form, as to them shall seem most likely to effect their Safety and Happiness. Prudence, indeed, will dictate that Governments long established should not be changed for light and transient causes; and accordingly all experience hath shewn, that mankind are more disposed to suffer, while evils are sufferable, than to right themselves by abolishing the forms to which they are accustomed. But when a long train of abuses and usurpations, pursuing invariably the same Object evinces a design to reduce them under absolute Despotism, it is their right, it is their duty, to throw off such Government, and to provide new Guards for their future security.--Such has been the patient sufferance of these Colonies; and such is now the necessity which constrains them to alter their former Systems of Government. The history of the present King of Great Britain is a history of repeated injuries and usurpations, all having in direct object the establishment of an absolute Tyranny over these States. To prove this, let Facts be submitted to a candid world.
There are various kind of elements that are present in periodic table. Some elements are harmful, some are radioactive, some are noble gases. Therefore, periodic table is the best platform to understand element.
What is periodic table?Periodic table is a table in which we find elements with properties like metals, non metals, metalloids and radioactive element arranges in increasing atomic number.
Periodic table help a scientist to know what are the different types of elements are present in periodic table so that they can discover the new elements that are not being discovered yet.
Periodic table is the best platform to have a look to all elements at once that are arranged in columns and groups mentioning their valences, electronegativity and Ionisation energy symbols.
Therefore, periodic table is the best platform to understand element.
Learn more about periodic table, here:
https://brainly.com/question/11155928
#SPJ2
Which one is it ?? Hurry please!!

Answers
Answer:
3-Ethyl-2,4-Dimethylhexane
Explanation:
3-ethyl-2,4-dimethylhexane
bp’s efforts to close the blowout preventer and install a containment dome following an explosion on the deepwater horizon drilling rig is an example of ________ change.
Answers
The British Petroleum (bp's) effort to close the blowout preventer and install a containment dome following an explosion on the deepwater horizon drilling rig is an example of regulatory policy change.
The deepwater horizon drilling rig is a semi-submersible, transportable, floating, flexibly oriented drilling rig that could work in sea depths of up to 10,000 feet which is approximately 3,000 meters.
During the explosion that occurred in April 2010, British Petroleum made several efforts to contain the damages made and to prevent further outbreaks of disasters.
Part of the changes was shifting from a blowout preventer that has a specialized valve to seal, manage, and monitor oil and gas wells in order to prevent blowouts to a containment dome (a crucial component of a system meant to control an oil well's underwater blowout).
Therefore, we can conclude that the British Petroleum (bp's) effort to close the blowout preventer and install a containment dome following an explosion on the deepwater horizon drilling rig is an example of regulatory policy change.
Learn more about deepwater horizon drilling rig here:
https://brainly.com/question/21103915?referrer=searchResults
In an experiment, 10.6 grams of steam is produced and then cooled. If the heat of vaporization is 2,257 joules/gram, how much energy is released after all the vapor turns to liquid?
Answers
Answer:
23,900 joules
Explanation:
plato answer
Answer:
23,900 joules
Explanation:
Edmentum
What is the molar mass of Mg(OH)2?
41 g/mol
42 g/mol
58 g/mol
82 g/ mol
Answers
Question 15 (1 point)
Iron (II) Bromide contains one iron atom and two Bromine atoms. Replacing the anion
with oxygen would require more or less oxygen atoms? Why?
Answers
Answer:
Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom. The result is that the total positive charge of the protons cancels out the total negative charge of the electrons so that the net charge of the atom is zero. Most atoms, however, can either gain or lose electrons; when they do so, the number of electrons becomes different from the number of protons in the nucleus. The resulting charged species is called an ion.
Cations and anions
When a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. The result is that the atom becomes a cation—an ion with a net positive charge.
The opposite process can also occur. When a neutral atom gains one or more electrons, the number of electrons increases while the number of protons in the nucleus remains the same. The result is that the atom becomes an anion—an ion with a net negative charge. We can illustrate this by examining some very simple cations and anions, those formed when a single hydrogen atom loses or gains an electron.
Note: Hydrogen is actually somewhat unusual in that it readily forms both cations and anions. Most elements much prefer to form only one or the other. In terms of its electron configuration, can you explain why hydrogen can form both cations and anions? Feel free to post in the comments at the end of the article!
A hydrogen cation, a hydrogen atom, and a hydrogen anion.
A hydrogen cation, a hydrogen atom, and a hydrogen anion.
Classification cation neutral atom anion
No. of protons 111 111 111
No. of electrons 000 111 222
Net charge 111++plus 000 111-−minus
If a neutral hydrogen atom ( \text{H}Hstart text, H, end text, center) loses an electron, it becomes a hydrogen cation ( \text{H}^+H
+
start text, H, end text, start superscript, plus, end superscript, left). Conversely, if the neutral \text{H}Hstart text, H, end text atom gains an electron, it becomes a hydrogen anion ( \text{H}^-H
−
start text, H, end text, start superscript, minus, end superscript, right), also known as a hydride ion. Image credit: adapted from Boundless Learning, CC BY-SA 4.0.
In the center column, we have a diagram of a single, neutral hydrogen atom. It contains one proton and one electron; thus, its net charge is zero. If hydrogen loses its electron, it forms the cation \text{H}^+H
+
start text, H, end text, start superscript, plus, end superscript (left column). The \text{H}^+H
+
start text, H, end text, start superscript, plus, end superscript cation has a net charge of 1+ from the one proton in the nucleus since there are no electrons to cancel out the positive charge. If neutral hydrogen gains an electron, it forms the anion \text{H}^-H
−
start text, H, end text, start superscript, minus, end superscript (right column). The \text{H}^-H
−
start text, H, end text, start superscript, minus, end superscript anion has a net charge of 1- because it has one extra electron compared to the total number of protons.
Explanation: Hopes this gives a better explanation on them!
Al Sections
The picture shows a boy roasting a marshmallow over a fire. Using the picture, which of the following is the BEST example of light energy
- The fire cooking the marshmallow
. The bow moving his stick near the fire
. The fire allowing the boy to see the area around him
The cracking sounds that the fire makes as it burns wood.
Answers
Answer:
answer number one I think?
Having problems figuring out molar mass in general. steps would be most appreciated.
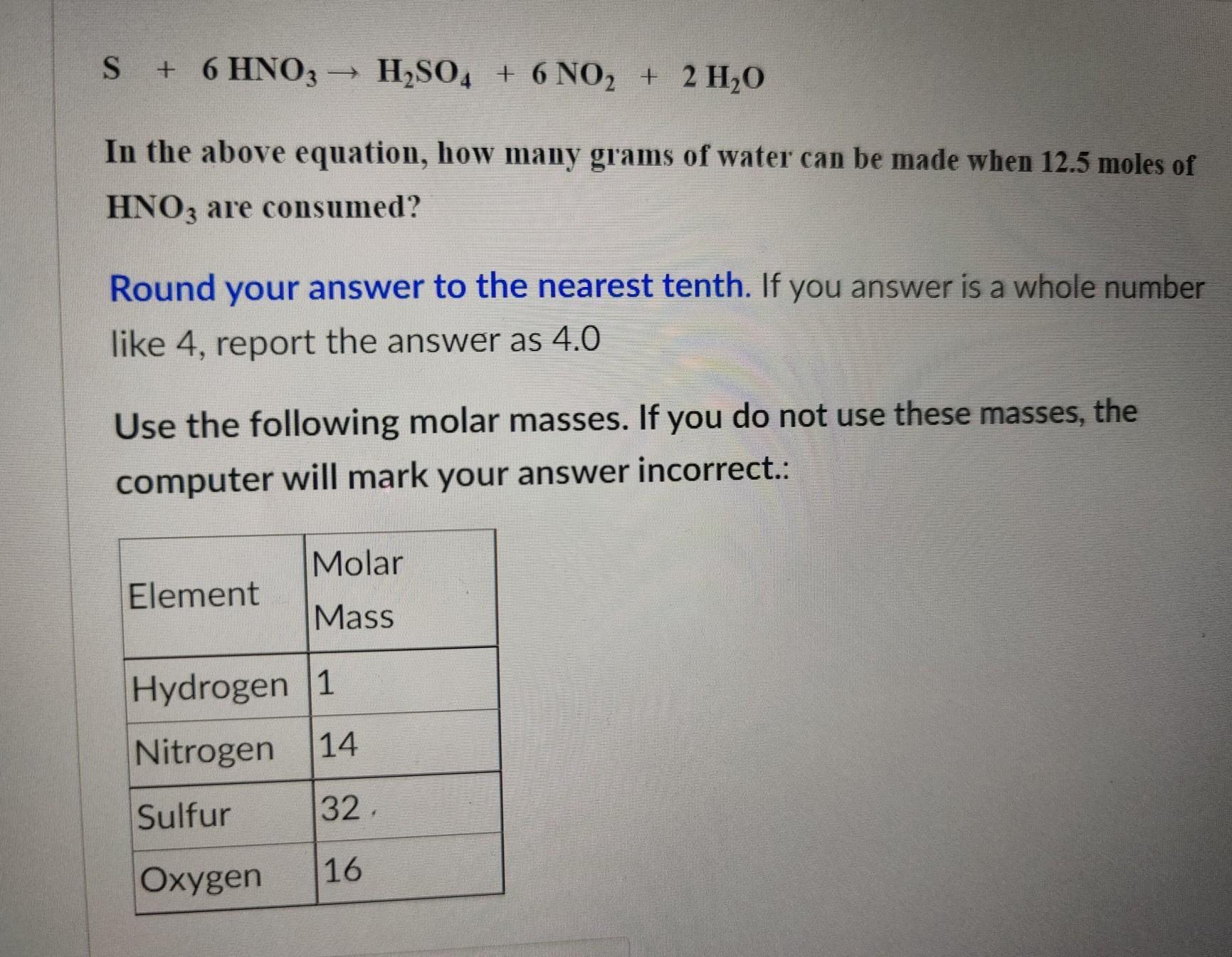
Answers
Answer:
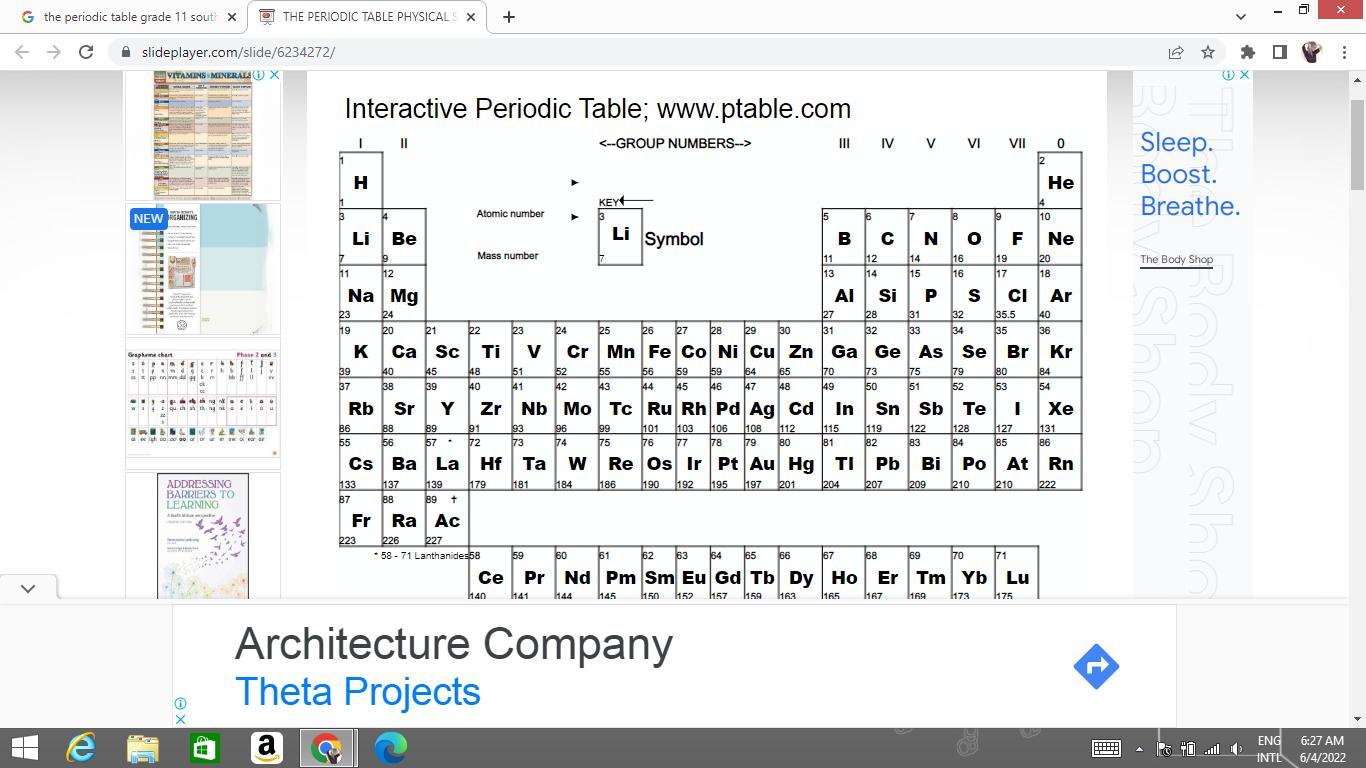
The periodic table
Explanation:
As you can see in the screenshot. Each element on the periodic table has an atomic number and a mass number. The atomic numbers are arranged in numerical order from right to left in the periodic table. The second number there is the atomic mass.
When doing stoichiometric ratios, you need to use the atomic mass of each element to determine the molecular/molar mass.
example. Find the molar mass of
H₂SO₄
Hydrogen (2) + sulphur (1) +oxygen (4)
= atomic mass of H(2)+ atomic mass S(1)+ atomic mass oxygen(4)
=1(2) + 32(1)+16(4)
=2+32+64
=98
N.B We do not use the co-efficient when looking for molar mass, we only use the atom and subscript
A 322 g sample of lead (specific heat = 0.138 J/gºC) is placed into 264 g of water at 25°C. If
the system's final temperature is 46°C, what was the initial temperature of the lead?
Answers
Answer:
-6.31°C
Explanation:
To solve this problem, we can use the principle of heat transfer:The heat gained by the lead (q_lead) equals the heat lost by the water (q_water).The formula to calculate heat transfer is:
q = m * c * ΔTWhere:
q = heat transfer
m = mass
c = specific heat
ΔT = change in temperatureFor the lead:
q_lead = m_lead * c_lead * ΔT_leadFor the water:
q_water = m_water * c_water * ΔT_waterGiven values:
m_lead = 322 g
c_lead = 0.138 J/gºC
ΔT_lead = T_final - T_initial_lead (unknown)
m_water = 264 g
c_water = 4.18 J/gºC (specific heat of water)
ΔT_water = T_final - T_initial_water = 46°C - 25°C = 21°CSince the heat gained by the lead is equal to the heat lost by the water, we can set up the equation:
m_lead * c_lead * ΔT_lead = m_water * c_water * ΔT_waterSubstituting the given values:
322 g * 0.138 J/gºC * ΔT_lead = 264 g * 4.18 J/gºC * 21°CSimplifying the equation:
44.436 J/ºC * ΔT_lead = 2325.12 J/ºCDividing both sides of the equation by 44.436 J/ºC:
ΔT_lead = 2325.12 J/ºC / 44.436 J/ºC ≈ 52.31°CFinally, we can find the initial temperature of the lead:
T_initial_lead = T_final - ΔT_lead
T_initial_lead = 46°C - 52.31°C ≈ -6.31°CTherefore, the initial temperature of the lead was approximately -6.31°C.
NEED HELP Please Will Choose Brainlest
Kinetic molecular theory is often used to describe conductors because as the ________ increase the thermal conductivity of the material increases.
atoms
collisions
weight
Answers
Answer:
B
Explanation:
Collisions
Answer:
Collisions
Explanation:
The questions talking about molecules so particles are moving around. Hope this helps
how do you find the molar mass of neon
Answers
sample of oxygen is collected over water at a total pressure of 692.2 mmHg at 17°C. The vapor pressure of water at 17°C is 14.5 mmHg. The partial pressure of the O2
Answers
677mmHg
Explanation:
In answering this question, we use Dalton's law of partial pressures which states that in a mixture of two or more non-reacting gases, the total pressure is equal to the sum of the partial pressures of the individual gases making up the mixture.
From the question, the gases are oxygen and water vapour.
Therefore the total pressure (\(P_T\)) is the sum of the partial pressure of oxygen (\(P_O\)) and the partial pressure of water vapour (\(P_{W}\))
\(P_T\) = \(P_O\) + \(P_{W}\) -----------------(i)
\(P_T\) = 692.2mmHg
\(P_{W}\) = 14.5mmHg
Substitute these values into equation (i) as follows;
692.2 = \(P_O\) + 14.5
\(P_O\) = 692.2 - 14.5
\(P_O\) = 677.7
Therefore, the partial pressure of oxygen is 677mmHg
An ion of the element oxygen (O) has an overall charge of 2−. Therefore, the number of electrons in this oxygen ion is
.
Answers
Considering the definition of atomic number, the number of electrons in this oxygen ion is 18.
Definition of atomic numberAll atoms are made up of subatomic particles: protons and neutrons, which are part of their nucleus, and electrons, which revolve around them. Protons are positively charged, neutrons are neutrally charged, and electrons are negatively charged (electrons).
The atomic number represents the number of protons present in the nucleus of an atom of any element. If the atom is electrically neutral, the number of electrons is equal to the number of protons.
Number of electrons in the oxygenThe atomic number of oxygen is 16. If it is a neutral atom, the number of protons and the number of electrons is 16.
Anions are negatively charged ions. They are formed as a consequence of the gain of electrons. Therefore, anions are characterized by having more electrons than protons in their composition.
In this case, an ion of the element oxygen (O) has an overall charge of -2. It means that the neutral atom has gained 2 electrons, so it will have a number of electrons of 18.
Learn more about atomic number:
brainly.com/question/5527493
#SPJ1
how much energy is produced when 93.5 grams of oxygen react with 13.2 grams of hydrogen in the following reaction:
2H2+o2-->2 h2o triangleH=-572kJ
Answers
Answer:
-1670.24 kJ
QUICK Explanation:
ΔH * moles of limiting reactant = Energy produced
-572 kJ/mol * 2.92 moles of O2 = -1670.24 kJ
LONGER EXPLANATION :
2 H2 + O2 -> 2 H2O
ΔH * moles of limiting reactant = Energy produced
enthalpy change or heat of reaction formula
1. Calculate the number of moles of oxygen (O2):
Number of moles = mass / molar mass
Number of moles of O2 = 93.5 g / 32.00 g/mol
≈ 2.92 mol O2
2. Calculate the number of moles of hydrogen (H2):
Number of moles = mass / molar mass
Number of moles of H2 = 13.2 g / 2.02 g/mol
≈ 6.53 mol H2
3. Determine the limiting reactant:
According to the balanced equation,
2 moles of H2 react with 1 mole of O2.
Calculate the moles of O2 based on the moles of H2:
(6.53 mol H2) / (2 mol H2/O2) = 3.27 mol O2
we need 3.27 mol O2 to react with the available H2
BUT only have 2.92 mol of O2 available
O2 is the limiting reactant
4.
Calculate the heat given off by assuming the complete consumption of the limiting reagent
calculate the amount of energy produced using the given enthalpy change (ΔH):
Energy produced = ΔH * moles of limiting reactant
Energy produced = ΔH * moles of O2 reacted
Calculate the energy produced using the given enthalpy change (ΔH):
Energy produced = ΔH * moles of O2 reacted
= -572 kJ/mol * 2.92 mol
≈ -1670.24 kJ
Therefore, approximately -1670.24 kJ of energy is produced when 93.5 grams of oxygen react with 13.2 grams of hydrogen in the given reaction. Note that the negative sign indicates that the reaction is exothermic (energy is released).
chatgpt
which disney character am I thinking of: he has a green outfit and a green hat
Answers
i don't know peter pan??
Answer:
Peter pan. that's the answer
If you don't use your muscles, they will get weaker and smaller, which is called:
Answers
Answer:
The correct answer would be atrophy.
Explanation:
I hope this helps you:)
Will give 10 points.
Why does an atom have a neutral charge?
A It has equal numbers of electrons and protons.
B. The number of neutrons equals the number of protons and
electrons in the atom.
C. It has equal numbers of electrons and neutrons.
D. It has equal numbers of neutrons and protons.
Answers
Answer:
option A it has equal numbers of electrons and protons
Explanation:
electrons are negatively charged and protons are positively charged, so if the numbers of each are equal, then the atom will be neutral.
Answer:
A. it has equal numbers of electrons and protons
Explanation:
The overall charge of every atoms is neutral because the number of electrons and protons is the same. Protons are positively charged while electrons are negatively charged and so they cancel out.
A gardener decides to use only part of their garden in the spring. What will most likely happen first in the unused part of the garden?
Answers
Answer:
Growth of weeds and grasses
Explanation:
The part of the garden, probably left to rest by the Gardner, would start experiencing a legion of invasive plants. Giant weeds and grasses could be seen, and brambles and tree seedlings could also sneak in.
Some Gardeners would recommend the use of herbicides to restore order to an abandoned garden even though certain scientists are of the opinion that chemicals may not offer a sound solution to the problem.
When current is allowed to flow, which species is oxidized? A) Cr₂O₇²⁻ B) Cr³⁺ C) MnO⁴ D) Mn²⁺ E) H³⁺
Answers
When current is allowed to flow, the species that gets oxidized depends on the specific conditions of the system. However, based on the given options, the likely species to be oxidized would be A) Cr₂O₇²⁻.
When current flows in a system, oxidation and reduction reactions occur simultaneously at different electrode surfaces. The species that gets oxidized is the one that loses electrons. In the given options, Cr₂O₇²⁻ and MnO⁴ are both polyatomic ions with high positive oxidation states, while Cr³⁺ and Mn²⁺ have lower positive oxidation states. H³⁺ refers to a hydrogen ion, which is not a likely candidate for oxidation in this context.
In the case of Cr₂O₇²⁻, it has a higher positive oxidation state compared to Cr³⁺. Therefore, it is more likely to undergo oxidation and lose electrons when current is allowed to flow. The half-reaction for the oxidation of Cr₂O₇²⁻ to Cr³⁺ can be represented as follows:
Cr₂O₇²⁻ (aq) → 2Cr³⁺ (aq) + 3e⁻
This reaction involves the reduction of another species, which gains the electrons released during the oxidation of Cr₂O₇²⁻. It's important to note that the actual species being oxidized or reduced can vary depending on the specific conditions of the system, such as pH and the presence of other reactants.
To learn more about oxidized refer:
https://brainly.com/question/13892498
#SPJ11
Moving to another question will save this response. Question 7 140 Ba has a half-life of 283.2 hours. How long would it take for 35 mg of 140 OA. 1452.6 hours OB. 9912.0 hours OC. 1006.9 hours D. 3.6 hours Ba in a sample to decay to 1.0 mg? TY NA
Answers
The total time required for the decay is approximately 1416.0 hours, which is closest to the given option C. 1006.9 hours.
Option (C) is correct.
To calculate the number of half-lives required for the decay. The half-life of 140 Ba is given as 283.2 hours.
First, we calculate the fraction of the original amount remaining after each half-life. Since the half-life represents the time it takes for half of the substance to decay, the fraction remaining after each half-life is 1/2.
To find the number of half-lives required to decay from 35 mg to 1.0 mg, we can set up the following equation:
(35 mg) * (1/2)ⁿ = 1.0 mg
Where 'n' is the number of half-lives.
Now, let's solve for 'n':
(1/2)ⁿ = 1.0 mg / 35 mg
(1/2)ⁿ = 0.02857
To find 'n', we can take the logarithm (base 1/2) of both sides:
n = log base 1/2 (0.02857)
Using the logarithmic property, we know that log base a (b) = log base c (b) / log base c (a):
n = log (0.02857) / log (1/2)
Using a calculator, we can find:
n ≈ 4.243
Since 'n' represents the number of half-lives, and we usually round up to the nearest whole number for half-life calculations, we get:
n ≈ 5
Therefore, it would take approximately 5 half-lives for the 35 mg sample of 140 Ba to decay to 1.0 mg.
To calculate the total time required, we multiply the half-life by the number of half-lives:
Total time = 283.2 hours * 5
Total time ≈ 1416 hours
Rounding to one decimal place, the total time required for the decay is approximately 1416.0 hours, which is closest to the given option C. 1006.9 hours.
To learn more about half-lives here
https://brainly.com/question/30599798
#SPJ4