How does a noncompetitive inhibitor affect the value of Km (Michaelis constant) of an enzyme?
Answers
A noncompetitive inhibitor affects the value of Km (Michaelis constant) of an enzyme by not altering the Km value.
Km is a measure of the affinity of an enzyme for its substrate, and a noncompetitive inhibitor binds to a different site on the enzyme, causing a decrease in Vmax (maximum velocity) but not affecting the binding of substrate to the active site.
Noncompetitive inhibitors bind to a different site on the enzyme other than the active site, which doesn't directly impact substrate binding affinity. However, they do decrease the overall enzyme activity and reduce the maximum reaction rate (Vmax).
Learn more about noncompetitive inhibitor: https://brainly.com/question/31067242
#SPJ11
Related Questions
Why are there more sodium ions in the sulfide compound
Answers
Answer:
2 Na
Explanation:
2 Na atoms per 1 Sulfide atom
hope this helps
T/F. the natural ph of hair is between 4.5 and 5.5.
Answers
True, the natural pH of hair is between 4.5 and 5.5.
The pH scale measures the acidity or alkalinity of a substance, ranging from 0 (highly acidic) to 14 (highly alkaline), with 7 being neutral. Hair and the scalp have a slightly acidic natural pH level, typically falling within the range of 4.5 to 5.5. This acidity helps maintain the hair's integrity and protect it from damage.
Hair has a natural pH level of 4.5 to 5.5, making it slightly acidic. This acidity is important for protecting the hair and maintaining its structural integrity. Balancing pH levels in hair care products is crucial for healthy hair and scalp.
The statement is true - the natural pH of hair lies between 4.5 and 5.5, contributing to the overall health and protection of hair and scalp. It is essential to consider pH levels when choosing hair care products to maintain hair health.
To know more about alkalinity visit:
brainly.com/question/31556972
#SPJ11
what is 750 grams of phosphorus
Answers
Answer:
750 g of phosphorus is 750 g of phosphorus
using the actual moles of o2 you determined from your experiment (n) and the theoretical moles of o2 you just calculated, show your calculations from experiment 1: ideal gas law - finding percent h2o2 with yeast for determining the percent hydrogen peroxide in your experimental sample.
Answers
Theoretical mass (O₂)= H₂O₂ Volume× H₂O₂ density× {( 1 mol O₂)/(2 mol H2O2)}×{( mol of H₂O₂ /g H₂O₂ )}
H2O2 Volume= 5 ml
Known density( H₂O₂ )= 1.02 g/ml
Molar mass ( H₂O₂ )= 34.01 g/mol
Reciprocal molar mass( H₂O₂ )= 0.0294
Theoretical mass( O₂)= 5×1.02× 0.0294× 1/2= 0.0749 moles
Actual moles of O₂ and percentage of H₂O₂
P= 753 mm Hg×1 atm/760 mm Hg= 0.991 atm
V= 45 ml× 1L /1000 ml= 0.045 L
n= PV/RT
Actual moles ( O₂)= (0.991×0.045)/(0.0821×296 K)= 0.0445/24.3016= 1.8×10^-3 moles
Percentage ( H₂O₂ )= Theoretical moles(O₂)×100= 0.0749×100= 7.49%
Mass of H₂O₂ (percent)= 3%
Concentration by mass( H₂O₂ )= 3 gm H2O in 100 ml of H2O
Volume ( H₂O₂ )= 100 ml
1 mol ( H₂O₂ )= 34.02 g O2
(3g H₂O₂ /100 ml Solution)×( 1 mol H₂O₂ /34.02 g H2O2)
No. Of moles ( H₂O₂ )= 0.088 moles
Mass ( H₂O₂ )= 0.088 moles × 34.02 = 2.99 g
% H₂O₂ = (2.99 g H₂O₂ /100 ml Solution)×100= 2.99%
% error= (% H₂O₂ from the bottle-experiment % H₂O₂ )/(% H₂O₂ from bottle)×100
=[ (3-2.99)/(3) ]× 100= 0.34% error.
Learn more about H₂O₂ here:- https://brainly.com/question/25566753
#SPJ1
the concentration of urea (mw = 60.0 g/mol) in a solution prepared by dissolving 16 g of urea in 39 g of h2o is ________ molal.
Answers
the concentration of urea in the solution prepared by dissolving 16 g of urea in 39 g of H2O is 6.85 mol/kg (or 6.85 molal).
To find the molality (molal concentration) of the solution, we first need to calculate the number of moles of urea in the solution.
Number of moles of urea = mass of urea / molar mass of urea
= 16 g / 60.0 g/mol
= 0.267 moles
Now, we need to calculate the mass of water in the solution in kg (since molality is expressed in terms of moles per kg of solvent).
Mass of water = 39 g / 1000 g/kg
= 0.039 kg
Therefore, the molality of the solution is:
Molality = number of moles of solute / mass of solvent in kg
= 0.267 mol / 0.039 kg
= 6.85 mol/kg
To learn more about molal concentration click here
brainly.com/question/31082216
#SPJ11
(Help ASAP) How many grams of Al2(SO4)3*18H2O are required to make 800 mL of a 0. 300 M solution?
can you show and explain?
Answers
We need 128.04 grams of Al2(SO4)3.18H2O to make 800 mL of a 0.300 M solution. Given that the volume of the solution (V) = 800 mL = 0.8 L
The molarity of the solution (M) = 0.300 M
We have to find out the mass of the compound Al2(SO4)3.18H2O required to make the solution.
To find the mass, we need to use the formula:
mass = molarity x molar mass x volume
Here, the molar mass of Al2(SO4)3.18
H2O = 666.39 g/mol (sum of the atomic weights of Al, S, O, and H)
Let the mass of the compound be x grams. Substituting the given values in the above formula:
mass = 0.300 x 666.39 x 0.8
= 160.05 x 0.8
= 128.04 g
To leran more about molarity, refer:-
https://brainly.com/question/30909953
#SPJ11
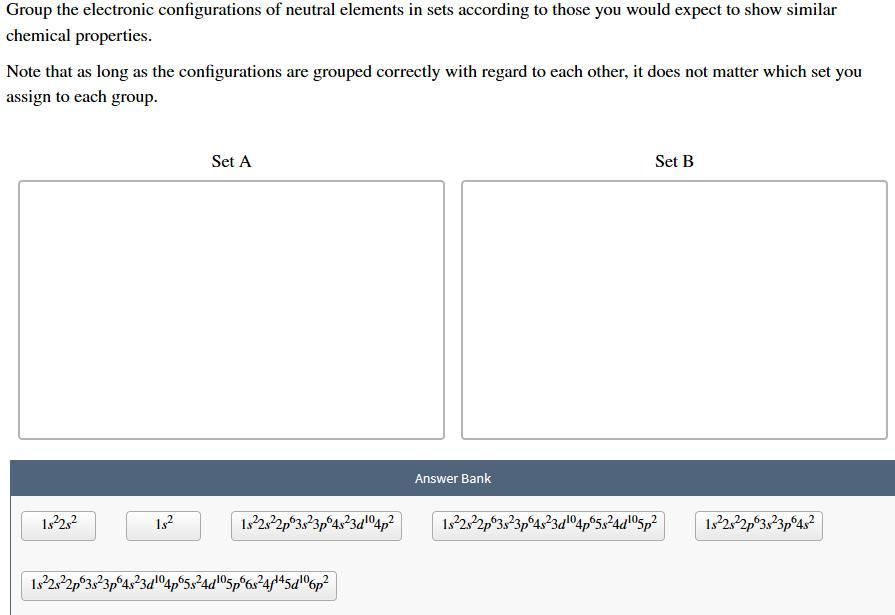
Group the electronic configurations of neutral elements in sets according to those you would expect to show similar chemical properties. Note that as long as the configurations are grouped correctly with regard to each other, it does not matter which set you assign to each group. Set A Set B Answer Bank 1:22:2 182 1922s22p03823p64323204p2 1322322p03823p 4323d"°4p65324d05p2 1922,22p3:23p6432 1.322s22p 3,23p64323 5p6s2445dº6p2
Answers
Set A elements behave similarly due to valence electrons in s and p orbitals, while Set B elements behave differently due to valence electrons in both s and d orbitals.
Set A:
1s²2s²
1s²
1s²2s²2p⁶3s²3p⁶4s²
Set B:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p²
The elements in Set A have valence electrons only in the s and p orbitals of their outermost energy level. This makes them similar in chemical behavior, as they tend to form covalent bonds by sharing electrons.
The elements in Set B have valence electrons in both the s and d orbitals of their outermost energy level, which gives them unique chemical properties, including the ability to form coordination complexes and exhibit variable oxidation states. Therefore, they are different in chemical behavior compared to the elements in Set A.
Learn more about neutral elements here: brainly.com/question/30433564
#SPJ4
Complete question is in the image attached below
describe the steps of mushroom farming
Answers
heyy!
Answer:
Mushroom farming, also known as mushroom cultivation, typically involves several key steps. Here's a general overview of the process:
Substrate Preparation: The first step is to prepare the substrate, which is the material on which the mushrooms will grow. Common substrates include materials like straw, wood chips, sawdust, or agricultural waste. The substrate needs to be properly prepared by pasteurization or sterilization to eliminate competing organisms and create favourable conditions for mushroom growth.
Inoculation: Once the substrate is prepared, it is inoculated with mushroom spawn. Spawn is a material containing mycelium, which is the vegetative part of the mushroom fungus. The mycelium acts as the "seed" for mushroom growth. The spawn is mixed or distributed throughout the substrate, either by hand or using specialized equipment.
Incubation: After inoculation, the substrate bags or containers are placed in a controlled environment with specific temperature, humidity, and lighting conditions. This stage is called incubation. During incubation, the mycelium grows and colonizes the substrate, forming a network of interconnected threads.
Casing: Once the mycelium has fully colonized the substrate, the next step is casing. Casing involves applying a layer of material on top of the colonized substrate, usually a mixture of peat moss and other additives. This casing layer provides a microenvironment that promotes the formation of mushroom fruiting bodies.
Fruiting: Following casing, the bags or trays containing the substrate and casing layer are moved to a fruiting room or chamber. The fruiting room is set to specific environmental conditions, including temperature, humidity, and lighting, which simulate the natural conditions required for mushroom growth. Under these conditions, small pinheads begin to form, which then develop into mature mushrooms.
Harvesting: Once the mushrooms have reached maturity, they are ready for harvest. Harvesting involves carefully plucking or cutting the mature mushrooms from the substrate. It is essential to handle the mushrooms gently to avoid damage.
Post-Harvest Management: After harvesting, the mushrooms are sorted, cleaned, and packaged for distribution. Proper post-harvest management, such as refrigeration or controlled storage conditions, may be necessary to maintain freshness and quality.
Crop Rotation and Maintenance: Following the harvest, the substrate may undergo a process called "crop rotation." This involves removing the spent substrate, disinfecting the growing area, and starting the process again with fresh substrate and spawning. Regular maintenance, including monitoring for pests and diseases, maintaining appropriate environmental conditions, and adjusting cultural practices, is crucial for successful mushroom farming.
It's important to note that specific mushroom species may have different cultivation requirements, and there may be variations in the process depending on the type of mushroom being grown. Additionally, mushroom farming can be done in various scales, from small-scale operations to large commercial farms, each with its own set of practices and equipment.
hope you found that helpful :))
What is the % yield if 4 moles of hydrogen is reacted with 3 moles of oxygen and produces 3 moles of water?
Answers
The balanced equation for the reaction between hydrogen and oxygen to form water is:
2H2 + O2 -> 2H2O
To react 4 moles of hydrogen with 3 moles of oxygen, we will also need 1.5 moles of oxygen.
Since we have enough hydrogen and not enough oxygen to react completely, we can calculate the theoretical yield of water produced.
4 moles of H2 will react to produce 2 moles of H2O and 1.5 moles of O2 will react to produce 0.75 moles of H2O. Therefore, the total theoretical amount of water produced is 2 + 0.75 = 2.75 moles of water.
percent yield = (actual yield / theoretical yield) x 100
percent yield = (3 / 2.75) x 100 = 109.09%.
So the percent yield is 109.09%. This means that 109.09% of the theoretical yield was actually produced, and the reaction was more efficient than expected.
It's worth noting that percent yield can't be more than 100% because it implies that more than the theoretical amount of product was produced, which is not possible. In this case, the percent yield is not a realistic value, therefore, the actual yield and the theoretical yield should be rechecked.
How are traits inherited?
Please explain thoroughly
Answers
inherited traits are passed from parent to offspring according to the rules of Mendelian genetics. Most traits are not strictly determined by genes, but rather are influenced by both genes and environment
What is the overall enthalpy change dhrxn for the system? -1,300 kj -300 kj 300 kj 1,300 kj
Answers
The overall enthalpy change i.e ΔH for the system is -1300 kJ.
Enthalpy change in a reaction is defined as the difference in the potential energy of the products and the potential energy of the reactants.
This is represented by ΔHreaction.
The reaction of enthalpy change is given as
ΔHreaction= ΔHproducts - ΔHreactants
where ΔHproducts is the potential energy of the products
=[(-200) + (-300)]kJ
= -500kJ
And, ΔHreactants is the potential energy of the reactants
=800KJ
Therefore, by putting these values ΔHreaction can be calculated as
ΔHreaction
= [-500-800] KJ
=-1300 KJ
Thus, the overall enthalpy change is -1300KJ
To know more about enthalpy change here
https://brainly.com/question/12200084
#SPJ4
please help me
law of conservation of matter facts or characteristics explain in your own words
Answers
I agree with the above answer.
8. What is the molecular mass of NH3?
Answers
the answer is 17.031 g/mol
Answer:
17.031 g/mol
Explanation:
hard to explanation
What is missing from the solubility graph shown on the right? A graph with temperature in degrees Celsius ranging from 0 to 100 in units of 20 on the horizontal axis and unidentified tick marks from 0 to 300 in increments of 50 on the vertical axis. An orange line curves upward from 0, 90 through 50, 125 to 100, 245.
Answers
Answer:
graph title, label for the y axis, and legend explaining the orange and blue colors
Explanation:
10 points Seved Carbon dioxide at 300 K and 1 atm is to be pumped through a duct with a 10 cm x 10 cm square cross-section at a rate of 250 kg/h The walls of the duct will be at a temperature of 450 K The exit CO2 temperature reaches 380 K Assuming steady operating conditions, and smooth surfaces of the duct, determine the following a Reynolds number (Re) b Nusselt number (Nu) e Convection coefficient (h) 4. Heat transfer rate (a) e Length of the duct (L)
Answers
a) The Reynolds number (Re) is a dimensionless quantity that represents the ratio of inertial forces to viscous forces in a fluid flow. It is calculated by multiplying the fluid's density, velocity, and characteristic length, and dividing it by the fluid's dynamic viscosity. In this case, the Reynolds number can be calculated as Re = (density * velocity * length) / dynamic viscosity.
b) The Nusselt number (Nu) is a dimensionless parameter used to determine the convective heat transfer coefficient. It is calculated by dividing the convective heat transfer coefficient (h) by the thermal conductivity of the fluid (k) and multiplying it by the characteristic length of the flow. In this case, the Nusselt number can be calculated as Nu = (h * length) / k.
The convective heat transfer coefficient (h) can be determined using correlations or experimental data specific to the flow conditions. The heat transfer rate (Q) can be calculated using the equation Q = h * area * (T_wall - T_fluid), where area is the cross-sectional area of the duct, and (T_wall - T_fluid) is the temperature difference between the wall and the fluid. The length of the duct (L) is not provided in the given information and cannot be determined based on the provided data.
In summary, the Reynolds number (Re) and Nusselt number (Nu) can be calculated using the given information and appropriate fluid properties. The convective heat transfer coefficient (h) can be determined using correlations or experimental data. The heat transfer rate (Q) can be calculated using the convective heat transfer coefficient and the temperature difference between the wall and the fluid. However, the length of the duct (L) cannot be determined with the provided information.
for such more questions on Reynolds
https://brainly.com/question/13348722
#SPJ8
Heeeelp please! Science gravity stuff pls help me

Answers
Gravity acts towards the center of the earth. Gravity can not be stopped
What makes gravity different from other forces?While other fundamental forces, such as electromagnetic force, can be either attractive or repulsive depending on the charges involved, gravity is always attractive. This means that any two objects in the universe with mass will be attracted to each other.
Every object in the universe with mass is affected by the force of gravity. This makes it a universal force that plays a key role in the behavior of the cosmos.
Learn more about garvity:https://brainly.com/question/4014727
#SPJ1
Why does every chemical reaction require a certain amount of activation energy?
A Energy is released when the reactants begin to react.
B. Energy lost to the environment during the reaction must be replaced.
C. Forming the activated complex requires energy.
D. The products have more potential energy than the activated complex.
E. The reactants have less potential energy than the products.
Answers
Answer: C
Explanation:
If the decay has a half-life of 10.2 years, what mass of 60.8g carbon-14 will remain after 20.4 years
Answers
Answer:
15.2 g.
Explanation:
After 10.2 years mass remaining = 60.8 * 1/2 = 30.4 g
After another 10.2 years it is 30.4 * 1/2 = 15.2 g.
the diagram shows a model of the nitrogen cycle. which role do plants play in the nitrogen cycle?


Answers
Answer
D
Explanation:
They take up usable forms of nitrogen found in soil
Answer:
d
Explanation:
i took the test
Explain how we know that charge is conserved in this
reaction: Li+ CI → Lici
Answers
Answer:
Charge is conserved due to the groups in which Lithium and Chlorine are located in the periodic table of the elements.
Explanation:
In the reaction \(Li + Cl - > LiCl\), we can examine the groups in which Li and Cl are found in the periodic table of the elements. Lithium appears in Group 1A, or the alkali metals group, indicating that it carries a charge of +1. Chlorine appears in Group 7A, or the halogen group, indicating that it carries a charge of -1. Because LiCl's constituent elements carry the same charges as previously mentioned, LiCl will have an overall charge of 0.
The chemical equation can then be rewritten as \(Li^{+} + Cl^{-} - > LiCl\), which, if looking at the individual charges of Li and Cl in lithium chloride, becomes \(Li^{+} + Cl^{-} - > Li^{+}Cl^{-}\). Adding the charges on the reactant and product sides of this chemical equation gives us zero in both locations, meaning that we have a charge of 0 on the reactant side and a charge of 0 on the product side. This indicates that charge is conserved in this reaction.
Another way to look at this is expressed in the valence electrons of Li and Cl. Li has an electron configuration of \(1s^{2}2s^{1}\), where the n = 2 electron shell has one of eight total electrons needed to fill the valence shell. This means that Li will easily lose one electron in order to have an electron configuration where the n = 1 electron shell is full, \(1s^{2}\), and become the \(Li^{+}\) ion. Similarly, Cl has an electron configuration of \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{5}\) (or \([Ne]3s^{2}3p^{5}\)), meaning that the n = 3 electron shell is one electron away from becoming complete. Cl will easily gain one electron to have the electron configuration \([Ne]3s^{2}3p^{6}\) (or \([Ar]\)) in order to have an electron configuration where the n = 3 electron shell is full, \(3s^{2}3p^{6}\), and become the \(Cl^{-}\) ion. Thus, when Li and Cl bond, Li will lose the electron \([1, 0, 0, +\frac{1}{2}]\) and transfer it to Cl, where it will become the electron \([3, 1, 1, -\frac{1}{2}]\), thus conserving charge, as there is an equal total number of electrons before and after the reaction.
For the chemical equilibrium A+2B↔2C, the value of the equilibrium constant, K, is 10. What is the value for the equilibrium constant for the reaction written in reverse? 2C↔A+2B
K=
a. 0.10
b. 100
c. 10
d. -10
e. 1
Answers
In this case, the original equilibrium constant, K, is 10, so the reciprocal is 1/10, which equals 0.10. Therefore, the value for the equilibrium constant for the reverse reaction, 2C↔A+2B, is 0.10. So the correct option is A.
It is important to note that the direction of a reaction and its reverse are not equal in terms of the rate of reaction or the value of the equilibrium constant. The forward and reverse reactions occur simultaneously and at the same rate, but the concentrations of the reactants and products are different, leading to a different value for the equilibrium constant for each direction.
In this case, the forward reaction is favored because the value of the equilibrium constant, K, is greater than 1, meaning there is a higher concentration of products compared to reactants at equilibrium.
To read more about chemical equilibrium, Visit-
https://brainly.com/question/8983535
#SPJ11
A sample of argon gas occupies 24.5 L at 345K and 0.980 atm. This sample contains how many moles of argon?
Answers
The number of moles of argon in the sample can be calculated by using the ideal gas law. The ideal gas law states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
We are given the volume, pressure, and temperature of the sample, so we can rearrange the equation to solve for n. n = PV/RT = (0.980 atm)(24.5 L)/(0.0821 atm•L/mol•K)(345K) = 7.37 mol.
Therefore, the sample of argon gas contains 7.37 moles of argon.
Learn more about gas at:
https://brainly.com/question/3637358
#SPJ1
Do you think one type of engineer is more important that others? Defend your
answer.
Answers
Answer:
No
Explanation:
All of the engineers today help us in many ways.
calculate each of the following quantities in 0.160 mol of C6H14O. calculate the number of atoms of H. calculate the number of atoms of C.
Answers
Answer:
To calculate the number of atoms of H and C in 0.160 mol of C6H14O, we need to first determine the number of moles of each element present in C6H14O.
The molecular formula of C6H14O shows that there are 6 carbon atoms, 14 hydrogen atoms, and 1 oxygen atom in each molecule of C6H14O.
The molar mass of C6H14O can be calculated as:
Molar mass of C6H14O = (6 × atomic mass of C) + (14 × atomic mass of H) + (1 × atomic mass of O)
= (6 × 12.01 g/mol) + (14 × 1.01 g/mol) + (1 × 16.00 g/mol)
= 86.18 g/mol
Therefore, 0.160 mol of C6H14O has a mass of:
Mass = molar mass × number of moles
= 86.18 g/mol × 0.160 mol
= 13.79 g
Now we can calculate the number of atoms of H and C in 0.160 mol of C6H14O.
Number of atoms of H:
Number of moles of H = 14 × 0.160 mol = 2.24 mol
Number of atoms of H = 2.24 mol × Avogadro's number
= 2.24 mol × 6.022 × 10^23/mol
= 1.35 × 10^24 atoms of H
Therefore, there are 1.35 × 10^24 atoms of hydrogen in 0.160 mol of C6H14O.
Number of atoms of C:
Number of moles of C = 6 × 0.160 mol = 0.96 mol
Number of atoms of C = 0.96 mol × Avogadro's number
= 0.96 mol × 6.022 × 10^23/mol
= 5.78 × 10^23 atoms of C
Therefore, there are 5.78 × 10^23 atoms of carbon in 0.160 mol of C6H14O.
Explanation:
What is the estimated effective nuclear charge, zeff, experienced by an electron in a 3p orbital of a chlorine atom?.
Answers
The estimated effective nuclear charge experienced by a 3 p electron of chlorine is 6.1.
It is the charge felt by any many-electron atom's outermost valence electrons. This is accomplished by taking into account the quantity of shielding electrons surrounding the nucleus.
It is used to assess the actual nuclear charge that an electron in a multi-electron atom experiences. This rule states that an electron's actual nuclear charge is less than its actual nuclear charge because of screening by other electrons in the atom.
The arrangement of an atom's electrons in its atomic orbitals is known as its electronic configuration. J. J. Thompson made the discovery of the electron. J. J. Thompson made the electron discovery in 1887. Every atom consists of a hefty nucleus that is encircled by electrons.
Learn more about electron:
https://brainly.com/question/26084288
#SPJ4
Which substance is likely to contain the highest percentage of double bonds in the hydrocarbon chains of its triglycerides?
Answers
Olive oil substance is likely to contain the highest percentage of double bonds in the hydrocarbon chains of its triglycerides.
In organic chemistry, hydrocarbons are organic compounds composed entirely of hydrogen and carbon. [1]:620 hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colorless and hydrophobic, and their odor is usually faint or exemplified by the liquid odor of gasoline or lighters. They come in a variety of molecular structures and phases.
Examples of hydrocarbons include gasoline, kerosene, lamp oil, and furniture oil. If someone accidentally drinks a hydrocarbon product that gets into their lungs, it can cause breathing problems. Severe injury or even death can result. Hydrocarbons are the primary energy storage molecules of all major fossil fuels (including coal, oil, and natural gas) and biofuels. It is also a raw material in the manufacturing process of many types of plastics.
Learn more about hydrocarbon here
https://brainly.com/question/3551546
#SPJ4
5.79 A 29.3-g sample of Ti reacts with O2 to form 48.9 grams of product. Determine the empirical formula of the product. 5.82 A compound composed of carbon, hydrogen, and oxygen is found to have an empirical formula of CH40. Determine the molecular formula of the compound if its molar mass is 88.10 g/mol. tobar So 5.84 A compound containing carbon, hydrogen, and oxygen was found to be 55.80% and 37.18% O by mass. Determine the molecular formula of the compound if its molar mass is found to be 86.08 g/mol. 2 bra 83 87. Determine the mass (in g) of each compound that contains 2.97 102 N atoms and convert each mass to moles of compound. 2.97 x 10N atoms in Mass (g) of Compound: NO Mole of Compound: Mass (g) of Compound: (NH4O Mole of Compound: Mass (g) of Compound: AI(NO3) Mole of Compound: 84
Answers
The empirical formula of the product in question 5.79 can be determined by finding the ratio of the elements present.
Given that a 29.3 g sample of Ti reacts with O2 to form 48.9 g of product, we need to calculate the moles of Ti and O in the reaction. The molar mass of Ti is 47.87 g/mol, so the moles of Ti in the sample is:
moles of Ti = mass of Ti / molar mass of Ti
moles of Ti = 29.3 g / 47.87 g/mol = 0.612 mol
To find the moles of O, we can use the difference in mass between the sample and the product:
mass of O = mass of product - mass of Ti
mass of O = 48.9 g - 29.3 g = 19.6 g
The molar mass of O is 16.00 g/mol, so the moles of O in the product is:
moles of O = mass of O / molar mass of O
moles of O = 19.6 g / 16.00 g/mol = 1.225 mol
Now we can find the empirical formula by dividing the number of moles of each element by the smallest number of moles:
Empirical formula = Ti(0.612 mol) O(1.225 mol) = TiO2
Therefore, the empirical formula of the product is TiO2.
The given information provides the masses of titanium (Ti) and oxygen (O) in the reaction. By converting these masses to moles and comparing their ratios, we determine the empirical formula of the product to be TiO2. This means that the product contains one titanium atom and two oxygen atoms per formula unit.
Learn more about empirical formula here:
https://brainly.com/question/32125056
#SPJ11
(A)Ch3ch2ch2ch2oh + [o] =?
(B)CH3CH2CH(OH)CH3 + H/heat=?
(C) (CH3)2CH=CH2 + Br2/CCl4 =?
(D) cyclohexane + Br2/peroxide
Answers
Answer:
d
Explanation: i think
In the summer, young animals grow bigger to give them a chance of surviving the winter. How does this compare to how a plant responds to the change in season?
Young plants grow seeds.
Young plants go dormant.
Young plants grow bigger.
Young plants drop their leaves.
Answers
Answer:
B
Explanation:
They go into a state of dormancy like hibernation .
In this stage they do
Metabolism slow heart rate lower body tempratureWhat speed will you have to go from Chicago to Hackensack in 260 hours
Answers
Speed you will have to go from Chicago to Hackensack in 260 hours is 4.9Km/hr.
The pace at which an object's position changes in any direction is referred to as its speed. The distance travelled in relation to the time it took to travel that distance is how speed is defined. Since speed simply has a direction and no magnitude, it is a scalar quantity.Speed = distance / timeUnit of speed is Km/hr or m/sec. Speed is calculated by dividing the distance travelled by the amount of time it took to get there. Divide the distance by the speed to find the passing time. Multiply the speed by the time to find the distance.Given,
Time = 260 hours
distance between Chicago to Hackensack is 1277 km
So speed = 1277/ 260 = 4.9 Km/ hr
Therefore, speed required to go from Chicago to Hackensack in 260 hours is 4.9 Km/ hr.
Learn more about speed here:
https://brainly.com/question/13262646
#SPJ9