Hassium belongs to what
block of the periodic table
PLS ANSWER ASAP
Answers
Explanation:
Hassium belongs to d-block of the periodic table.
Related Questions
From the following symble,
(18/8) 0^-2
this atom contains 2 electrons?
O True
O False

Answers
Answer:
False
Explanation:
HELP ME ASAP!!!!!!!!!!!!!!!!!!!!!!!!!!

Answers
Answer:
Digestive
Explanation:
how does one determine a molecular formula from the epirical formula
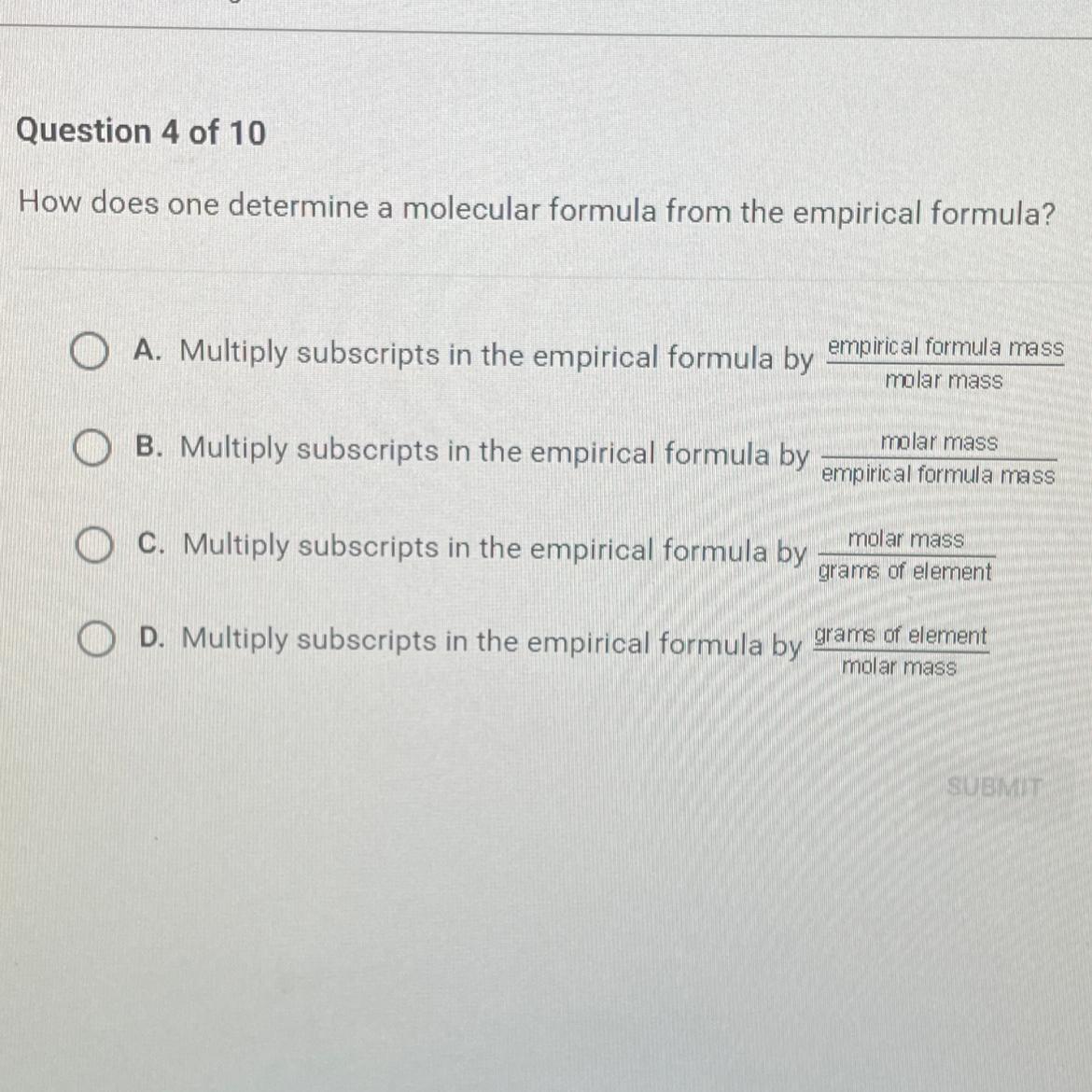
Answers
Answer:
C
Explanation:
consider a perceptron in rd. how many points can it shatter or more specifically what is the vc dimension of this perceptron? explain your answer
Answers
The VC dimension of a perceptron in ℝᵈ is d+1, which represents the maximum number of points it can shatter.
The VC (Vapnik-Chervonenkis) dimension is a measure of the capacity of a learning model, and in this case, we are considering a perceptron in ℝᵈ.
The VC dimension of a perceptron in ℝᵈ can be determined as follows: A perceptron is a linear binary classifier that separates input data into two classes using a hyperplane. In ℝᵈ, this hyperplane is a (d-1)-dimensional subspace. The VC dimension is the largest number of points that can be shattered, which means that the model can correctly classify all possible labelings of those points.
For a perceptron in ℝᵈ, the VC dimension is d+1. This is because any d+1 points in general position (i.e., not all lying on the same hyperplane) can be shattered by a perceptron. In other words, for every possible labeling of these d+1 points, there exists a hyperplane that can separate them into the two classes correctly. This can be shown through geometric reasoning or algebraic manipulation.
To further understand this, consider a perceptron in ℝ² (2-dimensional space). The separating hyperplane is a line, and the VC dimension is 3. Any set of 3 non-collinear points can be shattered by this perceptron, but if you try to shatter 4 points, you will find that it's impossible.
In conclusion, the VC dimension of a perceptron in ℝᵈ is d+1, which represents the maximum number of points it can shatter. This result helps us understand the capacity of the perceptron model and its limitations in learning more complex patterns.
for more such question on dimension
https://brainly.com/question/24514347
#SPJ11
assume there exists some hypothetical methat that exbihits ferromagnetic behavior and that has 1 simple cubic crystal strecutre, and atomic radius of 0.130 nm and a saturation flux density of 0.75 tesla. determine the number of bohr magnetons per atom for this material
Answers
When compared to a hypothetical ferromagnetic metal with a single simple cubic crystal structure, this substance possesses 1.14 bohr magnetons per atom.
Given that the cubic crystal atomic radius (r) is 0.130 nm
crystal structure's saturation flux density is equal to 0.75 tesla.
We are aware that n = M/N = B/0N.
n = BVc/0N, where BVc is the bohr magneton and Vc is the volume of the crystal.
N = 0.75x(2x0.130x10-9)3)/n = B(2r)3/0 (1.25x10-26x9.27x10-24)
n = 13.18x10-30/11.5x10-50 = 1.14x10^20
Therefore, this cubic crystal substance has 1.14 bohr magnetons per atom.
learn more about cubic crystal here:
https://brainly.com/question/27691721
#SPJ4
Medicine. A medical researcher is conducting a study to test the effectiveness of a drug designed to lower cholesterol levels. She randomly selects a sample of 100 males and 100 females. Sampling Methods. In Exercises 23-38, identify which of the following applies: simple random sample, systematic sample, convenience sample, stratified sample, or cluster sample. In each case, state whether you think the procedure is likely to yield a representative sample or a biased sample, and explain why.
Answers
The researcher used a stratified sample, which is likely to yield a representative sample by including both genders.
In this study, the medical researcher has employed a combination of sampling methods. For the selection of the 100 males and 100 females, a stratified sample has been used.
This method involves dividing the population into homogeneous subgroups (in this case, males and females) and randomly selecting participants from each subgroup. By using a stratified sample, the researcher ensures representation from both genders, which is likely to yield a more representative sample.
However, the specific sampling method used within each gender group is not mentioned. If the researcher employed a simple random sample within each gender group, it would involve randomly selecting participants from the entire population of males and females, respectively.
This would further enhance the representativeness of the sample, as each individual would have an equal chance of being selected.
It is crucial to note that the information provided does not specify the sampling method for selecting the participants. Therefore, it is challenging to determine whether convenience sampling, systematic sampling, or cluster sampling was employed.
However, these methods are generally considered less representative than simple random sampling or stratified sampling, as they may introduce bias by limiting the inclusion of certain individuals or groups.
In summary, the stratified sampling approach used to select 100 males and 100 females suggests an attempt to obtain a representative sample.
However, without further information on the specific sampling methods employed within each gender group, it is difficult to determine the overall representativeness or potential biases of the sample.
Learn more about Sampling
brainly.com/question/31890671
#SPJ11
What is the total number of electrons that can occupy the p sublevel?
Answers
it can hold up to 12 electrons.
explanation:
the p sub levels have 6 elements each, they each hold 2 electrons, so the entire p sub levels have 12 electrons.
Answer: 6 electrons
Explanation:
This is the maximum number of electrons that can occupy the p sublevel (I just took notes on it).
2. Assume that a class named Numbers has the following static member function declaration: static void showTotal(); Write a statement that calls the showTotal function.
Answers
The statement can be called using scope resolution operator.
Assuming that the showTotal() function is defined inside the Numbers class, the statement to call the function would be:
Numbers::showTotal();
The :: is the scope resolution operator, which is used to specify the namespace or scope of a function or variable. In this case, it specifies that the showTotal() function is a static member of the Numbers class. The function can be called without creating an instance of the class because it is declared as static.
learn more about scope resolution operator.
https://brainly.com/question/15554910
#SPJ11
How does static electricity work?
Answers
Answer:
"The phenomenon of static electricity requires a separation of positive and negative charges. When two materials are in contact, electrons may move from one material to the other, which leaves an excess of positive charge on one material, and an equal negative charge on the other."
Explanation:
Sited from Wikipedia
Balance these equations : ) ……H 2 + …..O 2 —> …. H 2 O…..FeCl 2(s) + ….. H 2 O (1)….> ….FeO (s) + …. HCl(aq)…..C 4 H 8(g) + …..O2(g)……> ….CO 2(g) + ….H 2 O (l)…..NaHCO 3(s) ….> ……Na 2 CO 3 + ….. CO 2(g) +…..H 2 O (g)…..NaOH (aq) + …….NgCl (aq) ….> …..NaCl (aq) + ….Mg(OH) 2(s)
Answers
In order to properly balance an equation, we need to make sure that the same amount of elements on the reactants side matches the number of elements on the products side, we can do that by increasing the number in front of each molecule, the so called stoichiometric coefficient. In the reaction from the question we can properly balance by adding the following stoichiometric coefficients
1. 2 H2 + O2 -> 2 HO2
2. FeCl2 + H2O -> FeO + 2 HCl
3. C4H8 + 6 O2 -> 4 CO2 + 4 H2O
4. ?
5. 2 NaOH + MgCl2 -> 2 NaCl + Mg(OH)2
Read this excerpt from the play An Adventure for Detective Dennis:
Stars twinkle in the windows of the library.
What element of a play is shown in this text?
Cast of characters
Dialogue
Scene
Setting description
Answers
Answer:
the answer is Scene
Explanation:
because it is describing the atmosphere of where the book/play is taking place
Answer:
the answer is Scene
Explanation:
N2(g) + 3H2(g) →2NH3(g)
At the end of the chemical reaction, 5 moles of NH3 are produced.
How many moles of N2 and H2 entered the reaction?
Answers
Answer:
2.5 moles of N₂ and 7.5 moles of H₂ entered the reaction
Explanation:
In reaction:
N₂(g) + 3 H₂(g) → 2 NH₃(g)
You can see that the stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) requires the following amounts of reagents and are produced:
N₂: 1 moleH₂: 3 molesNH₃: 2 molesThe following three rules can apply:
If 2 moles of NH₃ are produced from 1 mole of N₂ by stoichiometry of the reaction, 5 moles of NH₃ from how many moles of N₂ are produced?\(moles of N_{2} =\frac{5 moles of NH_{3} *1 mole of N_{2} }{2 moles of NH_{3}}\)
moles of N₂= 2.5
If 2 moles of NH₃ are produced from 3 moles of H₂ by stoichiometry of the reaction, 5 moles of NH₃ from how many moles of H₂ are produced?\(moles of H_{2} =\frac{5 moles of NH_{3} *3 mole of H_{2} }{2 moles of NH_{3}}\)
moles of H₂= 7.5
2.5 moles of N₂ and 7.5 moles of H₂ entered the reaction
expert that helps you learn core concepts.
See Answer
The molar solubility of PbBr2 at 25 C is 1.0 * 10^-2 mol/L. Calculate Ksp.
If 0.0490 g of AgIO3 dissolves per liter of solution, calculate the solubility-product constant.
Using the appropriate Ksp value from Appendix D in the textbook, calculate the pH of a saturated solution of Ca(OH)2.
Answers
The solubility product is 4x\(10^{-6}\) and 3x\(10^{-8}\) and the pH value is 12.35
The molar solubility of \(PbBr_{2}\) at 25 oC is 1.0 x \(10^{-2}\) \(\frac{mol}{L}\). to calculate the molar solubility = 1.0 x \(10^{-2}\) \(\frac{mol}{L}\)
\(PbBr_{2}- > Pb^{2}+2Br^-\)
Concentration of \(Pb^{+2}\)= \(1.10^{-2}\) \(\frac{mol}{L}\)
Concentration of \(Br^-\)= 2 x 1 x \(10^{-2}\) \(\frac{mol}{L}\) = 2 x \(10^{-2}\) \(\frac{mol}{L}\)
\(K_{sp}\) = [\(Pb^{+2}\)][\(Br^-\)]2 = [( 1 x \(10^{-2}\))( 2 x \(10^{-2}\))2] = 4 x \(10^{-6}\)
\(K_{sp}\) = 4 x \(10^{-6}\)
Now 0.0490 g of \(AgIO_3\) dissolves per liter of solution, and for the solubility-product constant.
We have, molar mass of \(AgIO_3\) = 283 g/mol
On solving
0.0490 g = \(\frac{0.049}{283}\)=1.731x \(10^{-4}\) moles
\(K_{sp}\) = [\(Ag^+\)][\(IO^{3-}\)] = (1.731 x \(10^{-4}\)) = 3 x \(10^{-8}\)
\(K_{sp}\) = 3 x \(10^{-8}\)
Now use the \(K_{sp}\) value from , for the pH of a saturated solution of \(Ca(OH)_{2}\).
\(Ca(OH)_{2} - > Ca^{+2}+2OH^{-}\)
\(K_{sp}\) = [\(Ca^{+2}\)][\(OH^-\)][\(OH^-\)] = s x \((2s)^2\) = 4\(s^{3}\)
5.5 x \(10^{-6}\)= 4\(s^{3}\)
s = 1.11 x \(10^{-2}\)M
[OH] = 2s = 2 x 1.11 x \(10^{-2}\) M = 2.22 x \(10^{-2}\) M
p(OH) = -log(OH) = -log(2.22 x \(10^{-2}\) M) = 1.65
pH = 14- pOH = 14- 1.65 = 12.35
pH = 12.35
Learn more about solubility product
brainly.com/question/1419865
#SPJ4
Scientists have changed the model of the atom as they have gathered new evidence. One of the atomic models is shown below. A purple center outlined in black with two concentric black circles around the center, the inner circle having 2 small green balls on it and the outer circle having 8 small green balls on it. What experimental evidence led scientists to change from the previous model to this one? A few of the positive particles aimed at a gold foil seemed to bounce back. The colors of light emitted from heated atoms had very specific energies. Experiments with water vapor showed that elements combine in specific proportions. Cathode rays were bent in the same way whenever a magnet was brought near them.
Answers
Answer:
B: The colors of light emitted from heated atoms had very specific energies.
Explanation:
dont know if its the same question i had on edg but i think its B. Sorry if i didnt understand the question :)
Answer:
b
Explanation:
rx: 0.7 l of 8% omeprazole suspension. your pharmacy stocks: 35% omeprazole suspension. how many ml of the 35% suspension would be needed for the dilution? (round to the nearest hundredth with no units!)
Answers
If the pharmacy stocks 35% omeprazole suspension, it required 160 ml of the 35% omeprazole suspension for the dilution.
It is required to apply the idea of dilution equations to determine the quantity of 35% omeprazole suspension required for dilution.
Let C₁ be the concentration of the 8% omeprazole suspension (8%), and let V₁ be the volume of the 0.7 L 8% omeprazole suspension.
Let C₂ be the concentration of the 35% omeprazole suspension, and let V₂ be the volume of the 35% omeprazole suspension that we need to find.
The dilution equation states that the product of the starting volume and concentration (V₁ × C₁) and the end volume and concentration (V₂ × C₂) should be identical.
V₁ × C₁ = V₂ × C₂
Putting the given values:
0.7 L × 8% = V₂ × 35%
0.056 L = V₂ × 35%
Dividing both sides by 0.35), get:
V₂ = 0.056 L / 0.35
V₂ = 0.16 L
Change 0.16 L to milliliters (ml):
0.16 L × 1000 ml/L = 160 ml
Thus, 160 ml of the 35% omeprazole suspension would be required for the dilution.
Learn more about dilution, here:
https://brainly.com/question/28202548
#SPJ4
What is the best set of reagents to achieve the following transformation? ph-co2h => ph-come
Answers
The best set of reagents to achieve the ph-co2h => ph-come will be Br2, CH2N2.
A material or ingredient supplied to a system to trigger a chemical reaction or check to see whether one occurs is known as a reagent as well as an analytical reagent. Reactant refers to a substance that is consumed during a chemical reaction. The phrases reactant and reagent just aren't frequently used interchangeably.
Diazomethane reacts swiftly and extremely effectively, producing just N2 as a byproduct, making it an appealing methylating agent for carboxylic acids including phenols.
Therefore, the best set of reagents to achieve the ph-co2h => ph-come will be Br2, CH2N2.
To know more about reagents
https://brainly.com/question/13127397
#SPJ4
Answer all the questions in the spaces provided
1. Name the elements present in the following compo
a. Sodium bromide (2mks)
b. Lead oxide (2mks)
Answers
Answer:
the volume of right circular cone is 5 liter calculate the volume of the two parts in to which the cone is divided by a plane parallel to the base one third on the way down from the vertex to the base give your answer to the nearest ml
The periodic table is divided into groups. In general,
Answers
The periodic table is divided into groups, which are columns representing elements with similar properties and electron configurations.
The periodic table is organized into groups or columns to classify elements based on their chemical and physical characteristics. Elements within the same group share similar properties because they have the same number of valence electrons, which determines their chemical reactivity. These groups are also known as families or vertical columns.
The periodic table consists of 18 groups, numbered from 1 to 18. Each group is labeled with a number and a letter designation, such as Group 1 (alkali metals) or Group 17 (halogens).
The elements within a group often display similar trends in atomic size, ionization energy, electronegativity, and chemical behavior. The grouping of elements helps scientists predict and understand the behavior of different elements based on their position in the periodic table.
For more questions like Periodic click the link below:
https://brainly.com/question/31672126
#SPJ11
Which describes how to calculate density?
mass divided by volume
volume divided by mass
mass added to volume
volume subtracted from mass
Answers
D=M/V
Density is equal to Mass over Volume
What is GMOs? ( Thanks btw )
Answers
Answer:
living organisms whose genetic material has been artificially manipulated in a laboratory through genetic engineering
Explanation:
How many elements are in this chemical formula?
2(NH4)2SO4
Answers
Answer: 30
Explanation:
Sulfur S 1
Oxygen O 4
Nitrogen N 2
Hydrogen H 8
15 in total. For 2 moles, 15*2 = 30
what functional group is naloxone
Answers
The functional group present in naloxone is amine.
What is functional groupA functional group is a group of atoms or bonds within a molecule that is responsible for the characteristic reactions of that molecule. In the case of naloxone, the amine functional group (-NH2) is present, which is a basic functional group containing a nitrogen atom bonded to two hydrogen atoms.
Naloxone is a medication used to reverse the effects of opioid overdose, and the presence of the amine functional group is important for its ability to bind to opioid receptors in the brain and block the effects of opioids.
In summary, the functional group present in naloxone is amine.
Learn more about naloxone at
https://brainly.com/question/28547819
#SPJ11
ANSWER QUICK PLEASE I GIVE BRANLIEST

Answers
Answer:
Answer 3 is the correct answer.
explain why the h-n-h angle in ammonia is smaller than the h-n-h angle in the ammonium ion.
Answers
The H-N-H angle in ammonia is smaller than the H-N-H angle in the ammonium ion due to the repulsion between the electrons in the NH4+ molecule and the tetrahedral geometry of the ammonium ion.
Ammonia (NH3) and ammonium ion (NH4+) have different geometries due to the presence of an additional hydrogen ion (H+) in the ammonium ion. The H-N-H angle in ammonia is approximately 107 degrees, while the H-N-H angle in the ammonium ion is approximately 109.5 degrees. This difference in the H-N-H angles can be explained by the following reasons:
1. Electron repulsion: In the ammonium ion, there is an extra hydrogen ion that carries a positive charge. This positive charge attracts the electrons in the NH4+ molecule, resulting in a smaller bond angle. As a result, the electron pairs are pushed closer together, which causes the H-N-H angle to increase slightly.
2. Tetrahedral geometry: The ammonium ion has a tetrahedral geometry, with four equivalent bonds and bond angles of approximately 109.5 degrees. This geometry is more stable and has lower energy than the trigonal pyramidal geometry of ammonia. The tetrahedral geometry of the ammonium ion is due to the sp3 hybridization of the nitrogen atom, which results in four hybrid orbitals that are oriented towards the four hydrogen atoms.
For such more questions on Ions
https://brainly.com/question/16934465
#SPJ4
How many grams of O2 gas are in a 6. 20 L container at a pressure of 897. 00 mmHg at 46. 40oC?
Answers
At 46.40 degrees Celsius and 897.00 mmHg, 6.20 liters of oxygen gas will contain a mass of 26.37 grams.
What is oxygen ?Oxygen is an element that is essential to life as we know it. It is a colorless, odorless, tasteless gas that makes up about 21% of the Earth's atmosphere. In its natural form, oxygen is made up of molecules of two oxygen atoms bonded together. Oxygen is vital to all living organisms, including humans, and is necessary for respiration and combustion. Oxygen plays a major role in many chemical processes, such as the burning of fuel, the corrosion of metals, and the formation of acids and bases. It is also an important component of environmental cycles, such as the water cycle and the carbon cycle.
897.00 mmHg = 897.00 mmHg × (1 atm / 760 mmHg) ≈ 1.17934 atm
46.40 °C + 273.15 = 319.55 K
Now, let's rearrange the ideal gas law equation to solve for the number of moles (n):
n = PV / RT
Plugging in the given values:
n = (1.17934 atm × 6.20 L) / (0.0821 Latm/molK × 319.55 K)
Simplifying the equation, we find:
n ≈ 0.2839 moles
To calculate the mass of O₂ gas, we need to multiply the number of moles by the molar mass of O₂:
Molar mass of O₂ = 32.00 g/mol
Mass of O₂ = 0.2839 moles × 32.00 g/mol ≈ 9.0928 g
Therefore, there are approximately 9.0928 grams of O₂ gas in the 6.20 L container at a pressure of 897.00 mmHg and a temperature of 46.40°C.
To learn more about oxygen
https://brainly.com/question/2111051
#SPJ4
what is the oxidation number of the central metal ion in each of the following complexes or compounds? [nicl3f]2− [fe(h2o)4(nh3)2]3 na[au(cn)2]
Answers
The oxidation number of the central metal ion in each of the given complexes or compounds can be determined by assigning oxidation numbers to the ligands and balancing the overall charge of the complex.
1. [NiCl3F]2-: The overall charge of the complex is -2. Chlorine has an oxidation state of -1, fluorine has an oxidation state of -1, and the oxidation state of nickel is x. Therefore, (-1 x 3) + (-1) + x = -2. Solving for x, we get the oxidation state of nickel as +2.
2. [Fe(H2O)4(NH3)2]3+: The overall charge of the complex is +3. Oxygen in water has an oxidation state of -2, nitrogen in ammonia has an oxidation state of -3, and the oxidation state of iron is x. Therefore, (-2 x 4) + (-3 x 2) + x = +3. Solving for x, we get the oxidation state of iron as +3.
3. Na[Au(CN)2]: The overall charge of the complex is 0 (since Na has a charge of +1 and [Au(CN)2] has a charge of -1). Cyanide has an oxidation state of -1, and the oxidation state of gold is x. Therefore, (-1 x 2) + x = 0. Solving for x, we get the oxidation state of gold as +1.
In summary, the oxidation number of the central metal ion in [NiCl3F]2- is +2, in [Fe(H2O)4(NH3)2]3+ is +3, and in Na[Au(CN)2] is +1.
To know more about oxidation visit :
https://brainly.com/question/16976470
#SPJ11
Reactions review please help

Answers
Gases begin to lose their ideal behavior under conditions of low temperature and/or high pressures. At wer temperatures and/or high pressures, interparticular attractions can begin to take place, which result in attractive forces between gas particles, which result in a diminished collision frequency and force on the container walls, which results in a gas pressure that is lower than would be expected from ideal-behaving gas. Therefore the PV/RT ratio that is normally equal to 1 for one mole of gas is now less than 1. At very low temperatures and/or very high pressures, the particles of gas become significant with respect to the gas volume due to repulsive forces between the particles, which results in a gas volume that is higher than would be expected from ideal-behaving gas. Therefore the PV/RT ratio that is normally equal to 1 for one mole of gas is now greater than 1. To adjust for this deviation from ideal behavior, the van der Waals equation below adjusts the pressure UP and the volume DOWN when considering the PV = nRT equation of state for non-ideal gas behavior: (P+n²a/V²) (V-nb) = nRT whers a and b are van der Waals constants Generally speaking, the larger the gas (the greater number of electrons, the greater the electron distribution, the more complex gas particle, and the stronger attractive forces between the particles), the larger the van der Waal constant a. The larger the particle volume, the larger the van der Waals constant b. Which statement(s) is/are true? I. Ideally, the molar volume of a gas is 22.414 L at 1 atm and 25°C. II. The van der Waals "a" is greater for SO₂(g) than for He(g). III. For most gases, the slope of the PV/RT vs. Pext plot is initially negative. IV. The value of the van der Waals "b" is an indication of the strength of the IMF's in the gas.
Answers
Ideally, the molar volume of a gas is 22.414 L at 1 atm and 25°C (I). The van der Waals "a" is greater for SO₂(g) than for He(g) (II) due to stronger attractive forces in SO₂. For most gases, the slope of the PV/RT vs. Pext plot is initially negative (III) because interparticular attractions lower gas pressure.(I,II,III)
Statement I is true based on the ideal gas law. Statement II is true because SO₂ is a larger, more complex molecule with stronger attractive forces, resulting in a larger van der Waals constant "a."
Statement III is true as interparticular attractions at low temperature and high pressure decrease collision frequency, lowering gas pressure. Statement IV is incorrect because the van der Waals "b" constant indicates the particle volume, not the strength of intermolecular forces..(I,II,III)
To know more about intermolecular forces click on below link:
https://brainly.com/question/31797315#
#SPJ11
If an atom has 4 protons and 2 electrons its charge will be:
Answers
Answer:
It's charge will be positive
Explanation:
4>2
explain why temperature is not as hot during the summer when a city is on a body of water (for example San Diego vs. Imperial Valley).
Answers
Answer:
because the water brings a cool breeze when the wind blows
Explanation: