Find the change in wavelength for photons scattered through 180 degrees by free photons. Compare with the corresponding shift for electrons.
Answers
The change in wavelength for photons scattered through 180 degrees by free photons is known as Compton scattering. In Compton scattering, the wavelength of the scattered photon increases compared to the incident photon.
The change in wavelength for photons scattered through 180 degrees by free photons is given by the Compton scattering formula:
Δλ = λ' - λ = h / (m_ec) * (1 - cosθ)
where Δλ is the change in wavelength, λ' is the wavelength of the scattered photon, λ is the wavelength of the incident photon, h is Planck's constant, m_e is the electron mass, c is the speed of light, and θ is the scattering angle (180 degrees in this case).
On the other hand, the corresponding shift for electrons is described by the de Broglie wavelength formula:
Δλ = h / (p_e)
where Δλ is the change in wavelength, h is Planck's constant, and p_e is the momentum of the electron.
Comparing the two, we can see that the change in wavelength for photons in Compton scattering depends on the scattering angle and the electron mass, while the change in wavelength for electrons is solely dependent on their momentum. Therefore, the behavior and magnitude of the wavelength shift differ between photons and electrons in scattering processes.
To learn more about Compton scattering refer here:
https://brainly.com/question/29306626#
#SPJ11
Related Questions
Please help me this is my fourth attempt.


Answers
Explanation:
CH4 + 4S ---> CS2 + 2H2S
4) 0.75 mol S × (1 mol CS2/4 mol S) = 0.19 mol CS2
5) 3 mol H2S × (1 mol CH4/2 mol H2S) = 1.5 mol CH4
Fe2O3 + 2Al ---> 2Fe + Al2O3
6) 25 g FeO3 × (1 mol Fe2O3/159.69 g Fe2O3) = 0.16 mol Fe2O3
0.16 mol Fe2O3 × (2 mol Al/1 mol Fe2O3) = 0.32 mol Al
0.32 mol Al × (26.98 g Al/1 mol Al) = 8.6 g Al
7) Given:
45 g Al × (1 mol Al/26.98 g Al) = 1.6 mol Al
85 g Fe2O3 ×(1 mol Fe2O3/159.69 g Fe2O3)
= 0.53 mol Fe2O3
Let's look at how much Fe each reactant will produce:
1.6 mol Al × (2 mol Fe/2 mol Al) = 1.6 mol Fe
0.53 mol Fe2O3 × (2 mol Fe/1 mol Fe2O3) = 1.1 mol Fe
Note that the given amount of Fe2O3 will give us fewer Fe. Therefore, Fe2O3 is the limiting reactant.
8) Al will produce 1.6 mol Fe × (55.845 g Fe/1 mol Fe)
= 89 g Fe
Fe2O3 will produce 1.1 mol Fe × (55.845 Fr/1 mol Fe)
= 61 g Fe
9) Since Fe2O3 is the limiting reactant, the ideal yield of Fe for the reaction is 61 g. If the actual reaction only gave us 25 g Fe. then the percent yield of Fe is
%yield = (25 g Fe/61 g Fe) × 100% = 41%
10) If we only got 25 g Fe, then the amount of Al actually used in the reaction is
25 g Fe × (1 mol Fe/55.845 g Fe) = 0.45 mol Fe
0.45 mol Fe × (2 mol Al/2 mol Fe) = 0.45 mol Al
0.45 mol Al × (26.98 g Al/1 mol Al) = 12 g
Therefore, the leftover amount of Al is
25 g Al - 12 g Al = 13 g Al
When a company is to calculate the selling price of their products, they set up a product calculation. There are five benefits to the business when making a product calculation. Explain these five benefits.
Answers
Answer:
The pricing strategy is an important element in setting up the selling price of a product.
Explanation:
The pricing strategy is an important consideration while fixing up the selling price of product manufactured. When setting up a selling price of a product, the companies set up product calculation. Businesses should decide the pricing strategy before they advertise the products to the customers.
There are mainly 5 benefits to the businesses while doing a product calculation. They are:
--- Doing competition based pricing enables the company to compete with the rival companies product and is based on the market based study. Competition pricing is a useful tool for the retailers as well as the small businesses.
--- Doing a cost plus pricing helps the total cost of making the product and also an add up in the market in order to determine the pricing of the product.
--- Dynamic pricing :
Dynamic pricing is a non static pricing. Dynamic pricing is an efficient method for the market based on the supply and demand.
--- Penetration pricing :
Penetration pricing is used by the large companies which is used to capture the market share by the setting product prices at the below market level so as to gain customers.
--- Doing a research for the price skimming helps the company to set up the accurate price for the product rather than readjusting the prices of the product later on based on the demand and the supply.
which reagents can be used to convert an aldehyde to a carboxylic acid
Answers
To convert an aldehyde to a carboxylic acid, oxidation of the aldehyde functional group is required.
There are several reagents that can be used for this conversion:
1. Strong Oxidizing Agents:
- Potassium permanganate (KMnO4): In the presence of acidic conditions, KMnO4 can oxidize aldehydes to carboxylic acids.
- Chromic acid (H2CrO4): It is a strong oxidizing agent that can convert aldehydes to carboxylic acids.
2. Tollens' Reagent:
Tollens' reagent, also known as silver mirror reagent, is a solution of silver nitrate (AgNO3) and ammonia (NH3) in water. It can oxidize aldehydes to carboxylic acids under mild conditions. It produces a silver mirror on the inner surface of the reaction vessel.
3. Jones Reagent:
Jones reagent consists of a solution of chromium trioxide (CrO3) in diluted sulfuric acid (H2SO4). It is a strong oxidizing agent that can convert aldehydes to carboxylic acids.
These are some commonly used reagents to convert aldehydes to carboxylic acids through oxidation. The choice of reagent may depend on factors such as reaction conditions, desired selectivity, and other functional groups present in the molecule.
To know more about aldehyde visit;
brainly.com/question/30459994
#SPJ11
Which mineral has been fortified in salt which has prevented its deficiency in the us?.
Answers
Answer:
This deficiency of iodine in the diet can be addressed by fortification of salt i.e. adding iodine to salt. Salt has been identified as an effective vehicle for iodine because it is consumed almost daily and universall
Explanation:
hope you understand
in an ionic bond, ________ lose valence electrons and become _________ charged
Answers
Answer:
in an ionic bond, atoms lose valence electrons and become positively charged
Explanation:
To clarify, the atom that loses the electrons becomes a positively charged ion (cation), and the atom that gains them becomes a negatively charged ion (anion).
Please help! Its world science!
1. If you turn on the burner on a gas stove under a pan of cold water, energy moves from the burner to the pan of water. What is this type of energy transfer called?
2. If you burn wood in a fireplace, which type of energy resource are you using?
3. Which form of energy is an important factor in making electricity from water power?
4. What is a hydrocarbon?
5. What byproduct of nuclear energy has caused concerns about the use of this resource and why?
6. Which chemical element exposed in surface coal mining can cause environmental problems in nearby bodies of water?
7. What is the original source of most energy used on Earth?
8. When we burn a fuel, what is released that allows work to be done?
9. For biomass, solar, coal, natural gas, oil, and geothermal energy, identify each energy resource as renewable or non-renewable.
10. What factors are important in judging how helpful an energy resource is to us?
Answers
Energy as the ability to do work.
What is energy?
Energy has been defined as the ability to do work. Modern civilization is possible because people have learned how to change energy from one form to another and then use it to do work. People use energy to walk and for bicycle, to move cars along roads and boats through water, to cook food on stoves, to light our homes and offices, to manufacture products.
There are different forms of energy, which may include:
HeatLightMotionElectricalChemicalGravitationalMagneticThese forms of energy can be grouped into two general types;
Potential (stored energy)Kinetic (working energy)Explanations
1. This is called convection.
2. You are using chemical energy and converting it to thermal (heat) energy.
3. Kinetic energy is the important factor
4. A hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline. Examples of hydrocarbons are coal, petroleum, natural gas and tar. They are used as energy sources.
5. Radioactive waste. These materials can remain radioactive and dangerous to human health for thousands of years.
6. Mercury.
7. The energy of the sun is the original source of most energy used on Earth.
8. Heat is released and this heat expended allows work to be done.
9.
biomass - renewablesolar - renewablecoal - non-renewablenatural gas - non-renewableoil - non-renewablegeo-thermal energy - renewable10.
It produces a lot of heat per unit mass.It does a huge amount of work per unit mass.It is easily accessible.It is easy to store and transport.It is economical.It produces less amount of smoke.Learn more about energy:https://brainly.com/question/13881533
#SPJ1
Which of the following is NOT an advantage that can only be gained by the use of digital data transmission instead of analog transmission?
A. Has less static
B. Carries more information
C. Takes up less bandwidth
D. Provides higher resolution images
Answers
Answer:
D. Provides higher resolution images
Explanation:
The advantages gained by the use of digital data transmission instead of analog transmission are;
1) Noise do not damage digital signal transmission but they impact on analog signals. Digital signals are therefore more secure than analog signals
2) The bandwidth required for digital signal transmission is much less than the bandwidth required for analog
3) Digital signal allow data transmission to very long distance
4) The rate of transmission with digital signals is higher with digital signals
5) Digital signals allow easy and cost effective methods by which they are replicated
The resolution of an image is a measure of the amount of pixels displayed per inch of the image, such that a high resolution image has more pixel per inch of the image. It is therefore a factor determined at the source of the image based on how much detail is required in an image before transmission
If the Ka of the conjugate acid is 4.83 × 10^-8 , what is the pKb for the base?
Answers
Explanation:
We are given: Ka = 4.83*10^-8
We use Ka to find pKa:
\(\begin{gathered} pKa\text{ = -log\lbrack Ka\rbrack} \\ \\ \text{ = -log\lbrack4.83}\times10^{-8}] \\ \\ \text{ = 7.32} \end{gathered}\)We know: pKa + pKb = 14
\(\begin{gathered} pKa+pKb\text{ = 14} \\ \\ \therefore pKb\text{ = 14-pKa} \\ \\ \text{ = 14-7.32} \\ \\ \text{ = 6.68} \end{gathered}\)Answer:
pKb = 6.68
How was the work of Newlands similar to that of Mendeleev on the periodic table?
They both arranged the elements in order of increasing atomic mass.
They both arranged elements that had similar properties into groups.
They both predicted the positions of undiscovered elements on the table.
They both placed the relative atomic mass of each element on the table.
Answers
Answer:
A. They both arranged the elements in order of increasing atomic mass.
Explanation:
Hope this helps! :)

The work of Newlands similar to that of Mendeleev on the periodic table was that they both arranged the elements in order of increasing atomic mass.
Hence, Option (A) is correct answer.
What is Newland's Law of Octaves ?Newland's Law of Octaves states that if the chemical elements are arranged in increasing order of atomic mass, every eighth element's properties are similar to that of the first element.
What is Mendeleev's Periodic Law ?Mendeleev's Periodic Law states that the chemical and physical properties of elements are a periodic function of their atomic masses.
Now, lets check all options one by one
Option (A): They both arranged the elements in order of increasing atomic mass.
Mendeleev arranged the elements in rows and column of a table in order of their increasing atomic mass. Newland arranged the elements in order of their increasing atomic mass that every eighth element's properties are similar to that of the first element.
So, it is correct option.
Option (B): They both arranged elements that had similar properties into groups.
Newlands arranged the elements that every eighth element's properties are similar to that of the first element. Mendeleev arranged the elements with similar properties in vertical column (group).
So, it is incorrect option.
Option (C): They both predicted the positions of undiscovered elements on the table.
Only Mendeleev predicted the position of undiscovered elements that is Gallium (eka-Aluminium) and Scandium (eka-Boron).
So, it is incorrect option.
Option (D): They both placed the relative atomic mass of each element on the table.
Only Mendeleev placed the relative atomic mass of each element on the table.
So, it is incorrect option.
Thus, we can say that The work of Newlands similar to that of Mendeleev on the periodic table was that they both arranged the elements in order of increasing atomic mass.
Hence, Option (A) is correct answer.
Learn more about the Periodic Table here: https://brainly.com/question/15987580
#SPJ2
3. Consider an iron-carbon alloy containing 0.60 wt% carbon. What is the proeutectoid phase? Compute the mass fractions of the proeutectoid phase and the pearlite phase. (15) arven C-0.60 knite chuse
Answers
The mass fraction of the pro eutectoid phase is approximately 0, and of the pearlite phase is approximately 1.
In iron-carbon alloy with 0.60 wt% carbon, the pro eutectoid phase is cementite (Fe₃C). To calculate the mass fractions of the pro eutectoid phase and the pearlite phase, consider the eutectoid reaction.
Eutectoid reactions in iron-carbon alloys are usually found at a composition of approximately 0.76 wt% carbon. As the alloy in question contains 0.60 wt% carbon it is hypo-eutectoid (i.e., below the eutectoid composition).
The lever rule will be used to calculate this equation as follows:
f₁ = \(\frac{C_{0} - C_{e} }{C_{1} - C_{e} }\)
where the values represent here :
f₁ = mass fraction of the pro eutectoid phase (cementite),
Cₒ =carbon content in the alloy (0.60 wt%),
Cₑ =eutectoid composition (0.76 wt%),
C₁ = carbon content in the cementite phase (6.70 wt% carbon).
After substituting the given values into the equation:
f₁ = \(\frac{0.60 - 0.76}{6.70 - 0.76} \\\)
f₁ = \(\frac{0.16}{5.94}\)
f₁ ≈ -0.027
Here the negative value of f₁ shows that there is no pro eutectoid phase present in the alloy. Rather, the entire alloy consists of the pearlite phase.
Hence , the mass fraction of the pro-eutectoid phase is approximately 0, and the mass fraction of the pearlite phase is approximately 1.
Learn more about alloy :
brainly.com/question/1759694
#SPJ4
In this activity, you will use a gas properties simulation to analyze how the variables that describe a gas relate to each other under different conditions. In the center of the simulation is a box with a lid. The box can be filled with gas particles. There’s also a thermometer and a pressure gauge. You can choose the type of particle you want. You can also change the energy of the particles by heating or cooling the box.
The controls on the right panel allow you to hold certain parameters constant or to change the number of particles in the box. Near the bottom are options to pause and reset the simulation.

Answers

Complete the following radioactive decay problem. Please help

Answers
Answer:
230 90Th
Explanation:
A careful observation of the equation given in question shows that 234 92U is undergoing alpha decay. This means that the resulting daughter nuclei will have a decrease of 4 in the mass number and a decrease of 2 in the atomic number.
Please see attached photo for further details.
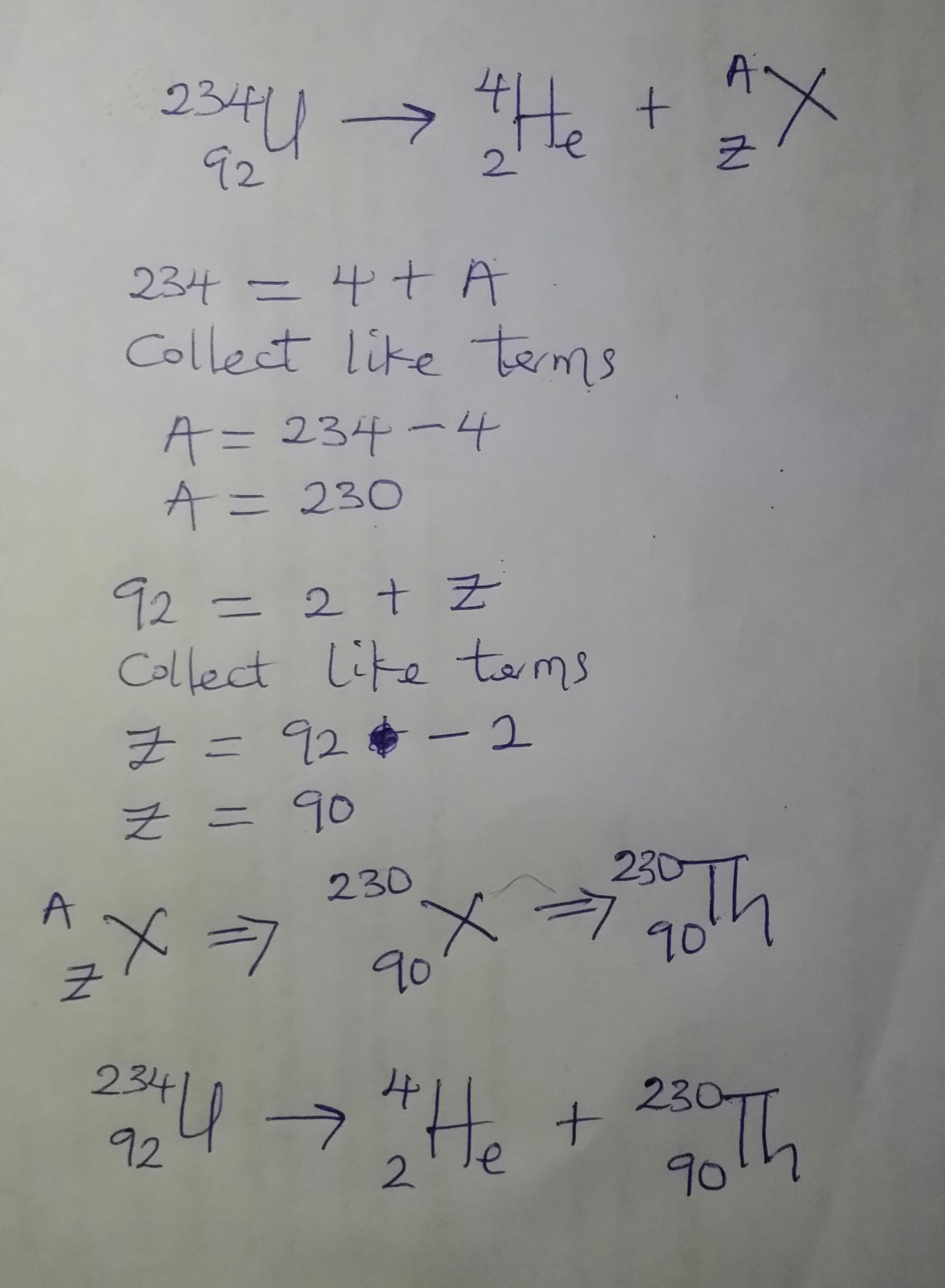
Adipic acid is an organic compound composed of 49.31%C, 43.79%O, and the rest hydrogen. If the molar mass of adipic acid is 146.1 g/mol , what are the empirical and molecular formulas for adipic acid?
Answers
Considering the definition of empirical and molecular formula, the empirical formula is C₃H₅O₂ and the molecular formula is C₆H₁₀O₄.
Definition of empirical formulaThe empirical formula or minimal formula is one that indicates the type of atoms that make up a compound, the minimum ratio of the integer number of atoms and not necessarily the exact number of atoms in it.
Definition of molecular formulaThe molecular formula is the actual formula of the molecule and indicates the types of atoms and the number of each type involved in the formation of the molecule.
This formula can be obtained from the empirical or minimal formula, as long as the molecular mass of the compound is known. That is, divide the estimated molecular mass divided by the molecular mass of the empirical form. With the multiple obtained, we proceed to multiply the subscripts of the empirical formula, thus obtaining the molecular formula of the compound.
Empirical formula in this caseIn this case, you know:
Carbon (C): 49.31%Oxygen (O): 43.79%Hydrogen (H): 6.9%Assuming a 100 grams sample, the percentages match the grams in the sample. So you have:
Carbon (C): 49.31 gramsOxygen (O): 43.79 gramsHydrogen (H): 6.9 gramsThen it is possible to calculate the number of moles of each atom in the molecule, taking into account the corresponding molar mass:
Carbon (C): 49.31 grams÷ 12 g/mole= 4.109 molesOxygen (O): 43.79 grams÷ 16 g/mole= 2.74 molesHydrogen (H): 6.9 grams÷ 1 g/mole= 6.9 molesThe empirical formula must be expressed using whole number relationships, for this the numbers of moles are divided by the smallest result of those obtained. In this case:
Carbon (C): 4.109 moles÷ 2.74 moles= 1.5 Oxygen (O): 2.74 moles÷ 2.74 moles= 1Hydrogen (H): 6.9 moles÷ 2.74 moles= 2.5The empirical formula must be expressed using whole number relationships, so:
Carbon (C): 1.5× 2= 3Oxygen (O): 1× 2= 2Hydrogen (H): 2.5× 2= 5Therefore the C: H: O mole ratio is 3: 5: 2
Finally, the empirical formula is C₃H₅O₂.
Molecular formula in this caseThe molar mass of adipic acid is 146.1 g/mol and the molar mass of empirical formula is 69 g/mole.
Dividing the estimated molecular mass divided by the molecular mass of the empirical form, you get:
146.1 g/mol÷ 69 g/mol ≅ 2
This means that in the molecular formula there are 2 unit formulas, so it is necessary to multiply the number of all atoms by 2:
Molecular formula= 2×C₃H₅O₂
Molecular formula= C₆H₁₀O₄
The molecular formula is C₆H₁₀O₄.
Learn more about empirical and molecular formula:
brainly.com/question/26388921
brainly.com/question/14853529
brainly.com/question/13058832
#SPJ1
the chemist adds m silver nitrate solution to the sample until silver chloride stops forming. he then washes, dries, and weighs the precipitate. he finds he has collected of silver chloride. calculate the concentration of iron(iii) chloride contaminant in the original groundwater sample.
Answers
The concentration of iron(iii) chloride contaminant in the original groundwater sample is (C1 × V1 / V) × 162.2 g/mol.
Given that the chemist adds m silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected of silver chloride. Let us calculate the concentration of iron(iii) chloride contaminant in the original groundwater sample.Calculating the concentration of iron(iii) chloride contaminant in the original groundwater sample
Here is the given information;
Mass of silver chloride precipitate = m grams
Volume of groundwater sample taken = V ml
Volume of AgNO3 solution used = V1 ml
Concentration of AgNO3 solution = C1
Molar Mass of AgCl precipitated = 143.5 g/mol
The molarity of AgNO3 solution is given as;
Molarity of AgNO3 = Number of equivalents / Volume of solution in liters
We know that 1 mole of AgNO3 gives 1 mole of AgCl, i.e., AgNO3 is equivalent to AgCl.Therefore, the number of equivalents of AgNO3 is the same as the number of equivalents of AgCl.
Number of equivalents of AgNO3 = C1 × V1
Number of equivalents of AgCl = m / 143.5 g/mol
Concentration of FeCl3 = (Number of equivalents of FeCl3 / Volume of sample in liters) × Molar mass of FeCl3
Number of equivalents of FeCl3 = Number of equivalents of AgNO3
Number of equivalents of FeCl3 = C1 × V1
Concentration of FeCl3 = (C1 × V1 / V) × Molar mass of FeCl3
Concentration of FeCl3 = (C1 × V1 / V) × 162.2 g/mol
Hence, the concentration of iron(iii) chloride contaminant in the original groundwater sample is (C1 × V1 / V) × 162.2 g/mol.
To know more about contaminant visit:
https://brainly.com/question/28328202
#SPJ11
A molecule that contains 6 carbon atoms with a single functional group that is an alcohol
Answers
The molecule that contains 6 atoms comprising a single functional group is Hexanol, under the condition that the given molecule is that of an alcohol.
Its molecules contain 6 carbon atoms. The finishing -ol states an alcohol (the OH functional group), and the hex- stem presents that there are six carbon atoms in the LCC. The OH group is assembled to the second carbon atom.
Functional groups are considered as specified groups of atoms within molecules that are the reason for characteristic chemical reactions of those molecules . Some examples of functional groups include alcohols, aldehydes, ketones, carboxylic acids, esters, ethers, halogens, amines and amides.
To learn more about functional groups
https://brainly.com/question/30483921
#SPJ4
The complete question
Name a molecule that contains 6 carbon atoms with a single functional group that is an alcohol
You have 28.5 g of iron shot that has a volume of 3.60 mL. From this information, calculate the density of iron.
Answers
Answer:
\(\boxed {\boxed {\sf \rho \approx 7.917 \ g/mL}}\)
Explanation
Density can be found by dividing the mass by the volume.
\(\rho=\frac{m}{v}\)
We know the iron sheet has a mass of 28.5 grams and the volume is 3.60 milliliters.
\(m= 28.5 \ g \\d= 3.60 \ mL\)
Substitute the values into the formula.
\(\rho=\frac{28.5 \ g}{ 3.60 \ mL}\)
Divide.
\(\rho = 7.91666667 \ g/mL\)
Let's round to the nearest thousandth.
The 6 in the ten-thousandth tells us to round the 6 to a 7.
\(\rho \approx 7.917 \ g/mL\)
The density of iron is about 7.917 grams per milliliter.
An instructor has a jar of sulfur that contains 16 grams. The students are asked how many sulfur atoms are in the jar. Four students give the following responses:
Arlo says, "There are 1/2 times Avogadro's number of sulfur atoms in the jar."
Bob says, "There are sixteen sulfur atoms in the jar."
Celine says, "There are two times Avogadro's number of atoms in the jar."
Delbert says, "There are sixteen times Avogardro's number of atoms in the jar."
With which, if any, of these three students do you agree:
Arlo,
Bob,
Celine,
Delbert, or
I don't think any of these students are correct
Answers
The student with the correct statement is Arlo.
How to determine the number of atomsFirst, we shall determine the mole of sulphur in the jar. This can be obtained as follow:
Mass of sulphur = 16 gramsMolar mass of sulphur = 32 g/moleMole of sulphur =?Mole = mass / molar
Mole of sulphur = 16 / 32
Mole of sulphur = 1/2 mole
Finally, we shall determine the number of atoms in the jar. This can be obotained as follow:
From Avogadro's hypothesis,
1 mole of sulphur = 6.02×10²³ atoms
Therefore,
1/2 mole of sulphur = 1/2 × 6.02×10²³ atoms
1/2 mole of sulphur = 1/2 × Avogadro's number
Thus, the number of sulphur atom is 1/2 times Avogadro's number
Therefore, Arlo is correct.
Learn more about number of atoms:
https://brainly.com/question/20712650
#SPJ1
Which cross section below best represents the crustal plate
motion that is the primary cause of the volcanoes and deep
rift valleys found at midocean ridges?
Key
Continental crust
Oceanic crust
Mantle
Direction of plate motion
Answers
The correct answer is option C) Oceanic crust.
What is the oceanic crust made of?
Oceanic Crust Oceanic crust, extending 5-10 kilometers (3-6 kilometers) beneath the ocean floor, is mostly composed of different types of basalts. Geologists often refer to the rocks of the oceanic crust as “sima.” Sima stands for silicate and magnesium, the most abundant minerals in oceanic crust.
What are the characteristics of oceanic crust?Oceanic crust is thinner and denser than continental crust. This is because it has been compressed by the weight of the oceans it carries above it. It is also much younger than the Continental crust, as it is usually less than 200 million years old.
Learn more about Oceanic crust here: brainly.com/question/20512106
#SPJ2
Which two statements describe how volcanic activity can affect Earth's crust?
A) A volcano are most likely to form near the center of tectonic plates
B) A volcanic mountain becomes smaller each time it erupts
C) A volcano can suddenly get taller every time it erupts
D) Hot magma from inside earth is released at weak points in the crust
Answers
Answer:
D
Explanation:
The weak crust of the earth does not hold together as well as strong crust.
Answer:
A and B was right in our test.
Explanation:
A was: A volcano can suddenly get taller every time it erupts
B was: Hot magma from inside Earth is released at weak points in the crust.
2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
How does separating CHEMICALS through DISTILLATION harm the environment ?
Explain in your own words
Answers
Explanation:
Distillation is the separation of mixture of two liquids which differ in boiling point. Through Distillation vapour that is formed spread thoroughly in our environment
how do you rewrite 4 5/7 as an improper fraction. in steps
please don't use files they don't work
Answers
Answer:
33/7
Multiply the denominator of the fraction by the whole number.
Add this result to the numerator of the fraction
answer becomes the numerator of the improper fraction.
Explanation:
Answer:
33/7
Explanation:
1. take the fraction and multiply the denominator by 4 to get 28
2. add the numerator to that... 28+5=32
an arctic weather balloon is filled with 24.6l of helium gas inside a prep shed. the temperature inside the shed is 7 degrees celsius. the balloon is then taken outside, where the temperature is 7 degrees celsius. calculate the new volume of the balloon. you may assume the pressure on the balloon stays constant at exactly 1 atm. round your answer to 3 significant digits.
Answers
The balloon's new volume is 24.6L. An arctic weather balloon being inflated using 24.6 litres of helium gas in a prep shed. The shed is seven degrees Celsius inside.
When the balloon is hauled outside, it is seven degrees Celsius outside. Any three-dimensional solid's volume is equal to how much room it occupies. One of these solids can be a cube, a cuboid, a cone, a cylinder, or a sphere. Chemical compounds are composed of a large number of comparable molecules (or molecular entities), which are composed of atoms from various elements bonded together by chemical bonds. Because of this, a molecule composed of atoms from a single element is not a compound.
v1/t1 = v2/t2
24.6/7 = v2/7
v2 = 24.6L
v1 = v2
Learn more about helium gas here
https://brainly.com/question/26408362
#SPJ4
The formula for propane is c3h8 what would you need to know to answer how much energy is required to combust propane vs gasoline
Answers
For propane combustion, the balanced equation is:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
This equation shows that one molecule of propane (C₃H₈) reacts with five molecules of oxygen (O₂) to produce three molecules of carbon dioxide (CO₂) and four molecules of water (H₂O).
For gasoline, the composition can vary, and the exact balanced equation would depend on the specific hydrocarbon components present in the gasoline.
Once you have the balanced equations, you would also need to know the standard enthalpy of formation (ΔH°f) values for the reactants and products involved in the combustion reactions. These values represent the change in enthalpy (energy) during the formation of one mole of a substance from its constituent elements, at standard conditions.
With the balanced equations and the ΔH°f values, you can apply the principles of thermochemistry and use the Hess's Law to calculate the energy released during the combustion reactions of propane and gasoline. The difference in energy released would indicate the difference in energy required to combust propane vs gasoline.
An electron in the ground state of an infinite potential energy well has an energy of 6.1 eV. How much additional energy, in eV, must be supplied for the electron to jump from the ground state to the first excited state?
Answers
The electron in the ground state of an infinite potential energy well has an energy of 6.1 eV. To jump from the ground state to the first excited state, the electron must be supplied with an additional energy of 3.4 eV.
The energy levels of an electron in an infinite potential energy well are quantized, meaning that they can only have certain values. The ground state energy is the lowest energy level, and the first excited state is the next highest energy level. The difference in energy between the two states is 3.4 eV.
To jump from the ground state to the first excited state, the electron must absorb a photon with an energy of 3.4 eV. The photon will be emitted when the electron falls back to the ground state.
To learn more about excited state here brainly.com/question/15413578
#SPJ11
a student uses a glue stick with an area of 4 cm3, putting
a pressure of 0.5 N/cm2 on her book. Calculate the force
she puts on the glue stick.
Answers
Answer:
So F=2N
Hope this helps.
Explanation:
P= F/A, where P is pressure, F is force, A is area.
So
P=F/A
0.5N/cm2 = F/4cm2 <--(do cross
2N=F multiplication,
4×0.5)
( And pls check on the unit of area u wrote, it should be (4cm2), not (4cm3) Unit of area is cm2.)
what is C2H8 ? please hurry
(CH4=methane)
(C2H2=acetylene)
Answers
Answer:
Carbon carbon | C2H8 - PubChem.
Explanation:
Brainliest plz :)
Answer:
Carbon carbon | C2H8
Explanation:
happy to help
How many moles are equal to 2.4 x 1023 formula units of sodium chloride?
Answers
Answer:
The answer is 0.4 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\ \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{2.4 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{2.4}{6.02} \\ = 0.398671096...\)
We have the final answer as
0.4 molesHope this helps you
Which elements are present in the compound aluminium nitrate?
Answers
Answer:
Aluminum nitrate is a salt composed of aluminum and nitric acid, belonging to a group of reactive chemicals - organic nitrate and nitrite compounds. The nitrate ion is polyatomic, meaning it is composed of two or more ions that are covalently bonded. This ion makes up the conjugate base of nitric acid.
Explanation:
Answer:
Aluminum nitrate: Is a salt composed of aluminum and nitric acid. Making this belong to a group of reactive chemicals which is an organic nitrate and nitrite compounds. The nitrate ion is polymaic. Polymaic means it is composed of two or more ions that are covalently bonded.
Explanation:
Hope This Helps
Have A Great Day
~Zero~
calculate the molarity of a kcl solution made by dissolving 28.4 g of kcl in a total volume of 500. ml.
Answers
Molarity of a KCL solution made by dissolving 28.4 g of KCL in a total volume of 500 ml is calculated as 0.7619 M KCL.
What is known as molarity?Mole is a unit of measurement used for chemical substance. Molarity is also known as the molar concentration of solution. It is the technique of calculating the amount of substance that a particular chemical solution contains.
As we know that the molar mass of KCl is 74.55 g/mol.
Hence, molarity = 28.4 g * 1 mol KCl /74.55 g * 1/500 mL * 1000 mL/1 L
Now, molarity = 0.7619 M KCl.
To know more about molarity, refer
https://brainly.com/question/26873446
#SPJ4