Fe2 O3 = Al =Fe Al2 O3
Answers
you will need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Aluminum + Iron (II) oxide
Related Questions
If 7.34 mol of O2 reacts, calculate the grams of CO2 produced.CH4 + 2O2—> CO2 + 2H2O
Answers
Answer:
\(161.48\text{ g}\)Explanation:
Here, we want to get the mass of carbon (iv) oxide produced
From the question, we have the balanced chemical reaction stating that 2 moles of oxygen molecule produced 1 mole of carbon (iv) oxide molecule
The number of moles of carbon (iv) oxide produced from 7.34 mol oxygen is thus:
\(\frac{7.34\times1}{2}\text{ = 3.67 moles}\)1 mole of carbon (iv) oxide contains 44 g
The mass in 3.67 moles will be:
\(44\times3.67\text{= 161.48 g}\)a buffer contains 0.250 m of weak acid hy and 0.110 m y-. what is the ph change after 0.0015 mol of ba(oh)2 is added to 0.210 l of this solution?
Answers
The pH of a buffer solution is determined by the concentration of the weak acid and the conjugate base present in the solution. When 0.0015 mol of Ba(OH)2 is added to 0.210 L of a solution containing 0.250 m of weak acid Hy and 0.110 m y-, the pH of the solution will be affected.
The amount of Ba(OH)2 added is small enough to not cause a drastic change in the pH, but it will still be higher than it was before the addition. The pH of the solution can be calculated by taking the ratio of Hy to y- and then using the Henderson-Hasselbalch equation, which states that the pH of a buffer solution is equal to the pKa of the weak acid plus the log of the ratio of the weak acid to the conjugate base.
After the addition of the Ba(OH)2, the ratio between Hy and y- will change, which will cause the pH to change as well. The exact pH of the solution can be calculated by taking into account the amount of Ba(OH)2 added, the initial concentrations of Hy and y-, and the pKa of the weak acid.
know more about buffer solution here
https://brainly.com/question/24262133#
#SPJ11
Can liquid CO2 exist on Earth? Why or Why not ?
Answers
I uploaded the answer to a file hosting. Here's link:
tinyurl.com/wpazsebu
what would be the symptoms for an air-cooled condenser where the air leaving the condenser is hitting a barrier and recirculating?
Answers
One thing that would be the symptoms for an air-cooled condenser where the air leaving the condenser is hitting a barrier and recirculating is: that there is less heat transfer from the refrigerant to the surrounding environment. Hence this will cause heat to accumulate in the condenser, making the cooling system inefficient.
What is a condenser?A condenser is a heat transfer used in heat transfer systems to cool a gaseous component and condense it into a liquid condition. The latent heat is released by the material and redistributed to the surrounding environment as a result.
The condenser's function is to accept high-pressure gas from the compressor and convert it to liquid. It does this by heat transfer or the notion that heat always moves from a warmer to a colder substance.
The condenser coil, evaporator, expansion valve, and compressor are the components. Each is critical to the overall performance of your unit.
Learn mroe about Condenser:
https://brainly.com/question/15563071
#SPJ1
If 247.65 grams of barium hydroxide react completely, how many grams of water should theoretically be produced?
Answers
If 247.65 g of barium hydroxide react completely, the grams of water produced theoretically should be 52.03 g.
The balanced chemical equation for reaction is :
2HNO₃ + Ba(OH)₂ → 2H₂O + Ba(NO₃)₂
To calculate number of moles, we will use the equation:
Number of moles = Given Mass g
Molar Mass g/mol
Moles of Ba(OH)₂ -
Number of moles = 247.65 g = 1.4454 moles
171.34 g/mol
Stoichiometry is defined as the number before chemical formula in a balanced reaction.
According to stoichiometry, the limiting reagent is barium hydroxide as it limits the formation of product. Yields can only be found using limiting reagent.
1 mole of Ba(OH)₂ produces = 2 moles of H₂O
Therefore, 1.4454 moles of Ba(OH)₂ requires = 2.891 moles
Mass of H₂O is = moles × molar mass
= 2.891 × 18 = 52.03 g
Thus, the theoretical yield produced is 52.03 g
To learn more about theoretical yield,
https://brainly.com/question/14980629
#SPJ4
The quantum number defines the shape of an orbital. spin magnetic principal angular momentum psi The subshell contains only one orbital. 5d 6f 4s 3d 1p The angular momentum quantum number is 3 in orbitals. s p d f a The principal quantum number of the first d subshell is 1 2 3 4 0 [Ar]4s^23d^104p^3 is the electron configuration of a(n) atom. As V P Sb Sn
Answers
The primary quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number are the four quantum numbers that make up an atom (ms).
There can be no zero for the main quantum number (n). Therefore, the permitted values for n are 1, 2, 3, 4, and so forth. Any integer between 0 and n – 1 can serve as the angular quantum number (l). For instance, if n = 3, l can be one of 0, 1, or 2. Any integer between -l and +l can be used as the magnetic quantum number (m). The principal quantum number is the first quantum number (n). The energy of an electron is substantially determined by the fundamental quantum number.
To know more about the quantum number,
https://brainly.com/question/2292596
#SPJ4
Chlorine gas occupies a volume of 25 mL at 300 K. What volume will it occupy at 600 K?
Answers
Chlorine gas occupies a volume of 25 mL at 300 K, 46 ml volume will it occupy at 600 K.
Initial volume V₁ = 25 mL
Final volume V₂ = ?
Initial temperature T₁ = 300 K
Final temperature T₂ = 600 K
To find out the final volume we will use the following equation.
V₁/T₁ = V₂/T₂
Rearrange it for V₂
Final volume V₂ = V₁/T₁ × T₂
Final volume V₂ = 23 ml / 300 ml × 600 K
Final volume V₂ = 0.0767
Final volume V₂ = 46 ml
46 ml volume will it occupy at 600 K.
You can also learn about Chlorine gas from the following question:
https://brainly.com/question/13123721
#SPJ4
Draw the lewis structure for each of the following - letter ba) NF3b) ClO3-c) HOBrd) SO3-2
Answers
Answer:
Explanation:
The question requires us to draw the Lewis structure for ClO3-.
In order to draw the Lewis structure of a molecule or ion, we need to consider the number of valence electrons in each atom of the structure:
O presents electron configuration: 1s2 2s2 2p4, thus it contains 6 valence electrons.
Cl presents electron configuration: 1s2 2s2 2p6 3s2 3p5, thus it contains 7 valence electrons.
Now, we can start drawing the Lewis structure for ClO3-.
1) First, we need to choose a central atom. Let's consider Cl as there is only one atom of it:
2) Next, we can "add" the electrons between the outer atoms and the central atom, representing bonds:
3) Now, let's complete the electrons on the atoms, starting with the outer atoms and then filling the central atom. Note that the total electrons is 3*6 + 1*7 = 25 electrons.



What is the molarity of 4 g of nacl in 3, 800 ml of solution? molar mass nacl = 58. 44 g/mol.
Answers
The molarity of the solution is 0.018 M.
For the calculation of molarity, we need to use the formula for molarity, put all values in the formula, and then complete the calculation.
formula of molarity= \(\frac{number of moles}{volume(L)}\) M
volume of solution is 3800ml or 3.8L
number of moles= \(\frac{Given mass}{Molar Mass}\) = \(\frac{4}{58.44}\) = 0.06844
where given mass is the mass of solute that are present in the solution
What is molar mass?Molar mass is also defied as the sum of atomic masses of all atoms present in the molecule.
molarity = \(\frac{0.06844}{3.8}\) = 0.018 M
Hence the molarity of solution is 0.018 M
Click on the following link to learn more about Molarity:
https://brainly.com/question/8732513#
#SPJ4
Consider the rate law below.R=k[A][B]^2What effect does doubling the concentration of each reactant have on the rate?-The rate increases to two times the original rate.-The rate increases to four times the original rate.-The rate increases to six times the original rate.-The rate increases to eight times the original rate.
Answers
Answer
The rate increases to eight times the original rate.
Explanation
When the concentration of each reaction is doubled, the rate increases by eighth compared to the initial rate.
Example: Rate =k[A][B]^2
Lets assume : [A]1 = 0.1 and [A]2 = 0.2
[B]1 = 0.2 and [B]2 = 0.4
Initially: Rate = (0.1)(0.2)^2 = 4x10^-3
After doubling: Rate = (0.2)(0.4)^2 = 0.032
Divide the final answer by the initial one
=> 0.032/4x10^-3 = 8
Which feature is most likely found at a divergent boundary?
Answers
Answer:
Most divergent plate boundaries are underwater and form submarine mountain ranges called oceanic spreading ridges. While the process of forming these mountain ranges is volcanic, volcanoes and earthquakes along oceanic spreading ridges are not as violent as they are at convergent plate boundaries.
Explanation:
Answer:
C-Fault block mountain
Explanation:
Tension occurs at divergent boundaries. Tesnion is a type of stress that occurs when plates move apart. Rocks stretch apart creating fault block mountains
The periodic table is divided into groups. In general,
Answers
The periodic table is divided into groups, which are columns representing elements with similar properties and electron configurations.
The periodic table is organized into groups or columns to classify elements based on their chemical and physical characteristics. Elements within the same group share similar properties because they have the same number of valence electrons, which determines their chemical reactivity. These groups are also known as families or vertical columns.
The periodic table consists of 18 groups, numbered from 1 to 18. Each group is labeled with a number and a letter designation, such as Group 1 (alkali metals) or Group 17 (halogens).
The elements within a group often display similar trends in atomic size, ionization energy, electronegativity, and chemical behavior. The grouping of elements helps scientists predict and understand the behavior of different elements based on their position in the periodic table.
For more questions like Periodic click the link below:
https://brainly.com/question/31672126
#SPJ11
Clarify the chemical reaction, and then write a balanced formula chemical equation
NaF
Answers
The balanced chemical equation for the formation of sodium fluoride, NaF is given below:
2 Na (s) + F₂ (g) ----> 2 NaF (s)
What kind of reaction is the formation of sodium fluoride?The formation of sodium fluoride, NaF is an example of a synthesis reaction.
In a synthesis reaction, two or more elements or compounds react together to form a single compound.
The formation of sodium fluoride is given in the chemical equation below:
2 Na (s) + F₂ (g) ----> 2 NaF (s)
The reaction is a highly exothermic reaction. The compound formed, NaF is an ionic compound.
During the reaction, two moles of sodium atoms lose an electron each to fluorine gas atom which readily accepts the electrons to fluoride ions.
electrostatic forces of attraction between the oppositely-charges ions keep the compound in a crystal lattice form.
Learn more about synthesis reactions at: https://brainly.com/question/26313963
#SPJ1
Reflect on any chemistry courses you might have taken before this class. (If you cannot recall a previous chemistry class, just think about how you have heard any chemistry concepts are discussed in your life.)
What concepts from those chemistry courses might be meaningful in helping you understand the human body?
Provide at least 2 examples and describe your thinking for why those chemistry concepts might apply to human anatomy and physiology.
What parts of the body might these concepts be helpful to understand?
My most recent topic in chemistry is: Hydrophobic effect phenomenon, but feel free to pick a topic that seems to be easier to you.
Answers
Concepts from chemistry, such as biochemical reactions and acid-base balance, can provide a foundation for understanding various aspects of human anatomy and physiology.
1. Biochemical Reactions: The study of biochemical reactions, including enzyme kinetics, can provide insights into the metabolic processes occurring in the human body. Understanding concepts like enzyme-substrate interactions, reaction rates, and enzyme regulation can help explain how cells produce and utilize energy, synthesize essential molecules, and carry out various physiological functions. This knowledge is applicable to understanding processes such as cellular respiration, digestion, protein synthesis, and hormone regulation.
2. Acid-Base Balance: The concept of acid-base balance, including pH and buffering systems, is crucial in maintaining homeostasis in the human body. Understanding how acids and bases interact and how the body regulates pH is essential for comprehending physiological processes like blood gas exchange, renal function, and acid-base disorders. This knowledge is particularly relevant in fields such as nephrology, respiratory medicine, and critical care.
The hydrophobic effect phenomenon has significance in biological systems. It plays a role in the folding of proteins, the formation of cell membranes, and the assembly of biomolecules. However, it may require a more detailed understanding of biochemistry and molecular biology to fully appreciate its implications in human anatomy and physiology.
Learn more about hydrophobic effect phenomenon here:
https://brainly.com/question/28481829
#SPJ11
compare the size of ions to the size of atoms from which they form
Answers
Cations are always smaller than the atoms from which they form. Anions are always larger than the atoms from which they form. Ions are usually bigger than the atoms from which they are formed.
When an atom receives or loses electrons, the atom's electron configuration changes, resulting in a net positive or negative charge.
This net charge expands the electron cloud surrounding the nucleus, making the ion bigger in size than the neutral atoms from which it arose. When a metal atom loses one or more electrons to create a cation, it shrinks in size because the positive charge of the nucleus pulls the remaining electrons more strongly.
When a nonmetal atom obtains one or more electrons to create an anion, it normally expands in size.Because of the increasing amount of electrons, the electron cloud surrounding the nucleus grows. It should be noted that this comparison is not absolute and is dependent on the individual factors involved. Some ions are smaller than their neutral atom counterparts, while others are similar in size.
learn more about atoms here:
https://brainly.com/question/29695801
#SPJ4
The complete question is:
Compare the size of ions to the size of atoms from which they form.
If 500.0 mL of 0.450 M sodium phosphate is reacted with an excess of iron (II) nitrate solution, how many grams of iron (II) phosphate are produced?
Answers
Answer:
If 500.0 mL of 0.450 M sodium phosphate is reacted with an excess of iron (II) nitrate solution, how many grams of iron (II) phosphate are produced?
idk
Explanation:
Answer:
164.2726 g
The ratio in the equation is 3:1 so the limit reaction ratio is 3:1 therefore, 0.392456 mol of were reaction.
Brainlist pls!
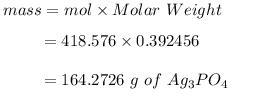
What do the elements Nickel and Technetium have in common?
Answers
Answer:
Both Nickel (Ni, 28, 58.69) and Technetium (Tc, 43, [98]) have these features in common:
transition metalssome gray color (Tc is silver)are in the d blockhave two electrons in valence shell (although Tc's valence is 6)very similar electronegativity levels (1.9 vs 1.91)don't have a refractive indexdon't have a critical temperaturedon't have a heat of combustion measurementdon't have an EU Number or NFPA hazard leveldon't have an autoignition point or a flashpointThere are various kind of elements that are present in periodic table. Some elements are harmful, some are radioactive, some are noble gases. Therefore, nickel (Ni) and technetium (Tc) belongs to d block element.
What is periodic table?Periodic table is a table in which we find elements with properties like metals, non metals, metalloids and radioactive element arranges in increasing atomic number.
Periodic table help a scientist to know what are the different types of elements are present in periodic table so that they can discover the new elements that are not being discovered yet.
Both Nickel (Ni) and Technetium (Tc) have these features in common:
Both are transition metals that is belong to d block
Both have two electrons in valence shell
Electronegativity value of these elements are same
Therefore, nickel (Ni) and technetium (Tc) belongs to d block element.
Learn more about periodic table, here:
https://brainly.com/question/11155928
#SPJ2
the solubility of o2 in water is 0.590 g/l at an oxygen pressure of 14.8 atm, at a particular temperature. what is the henry's law constant for o2?
Answers
The Henry's law constant for O2 at the given temperature is 0.0399 g/L/atm.
What is the partial pressure of a gas?Henry's law relates the concentration of a gas in a solution to the partial pressure of the gas above the solution. It is expressed by the equation:
C = kH * P
where C is the concentration of the gas in the solution, P is the partial pressure of the gas above the solution, and kH is the Henry's law constant.
In this problem, we are given the solubility of O2 in water at a pressure of 14.8 atm, which is 0.590 g/L. To find the Henry's law constant for O2, we need to rearrange the equation above to solve for kH:
kH = C/P
We can substitute the given values into this equation to get:
kH = 0.590 g/L / 14.8 atm
kH = 0.0399 g/L/atm
Therefore, Henry's law constant for O2 at the given temperature is 0.0399 g/L/atm.
Learn more about Henry's law
brainly.com/question/30636760
#SPJ11
Qué sucede al disolver una tableta de ALKA-SELTZER en agua caliente?
Answers
Answer:
FREE POINTS CUZ I DON'T UNDERSTAND
Explanation:
1) the average rate of reaction in the first minute
2)the average rate of reaction in the second minute
3)the time when the reaction has completed
4)the average rate of reaction for overall reaction
5)the rate of reaction at 30 seconds
6)the rate of reaction at 105 seconds

Answers
1. 0+15+30+45+60=150/5=30 s
0+10+16+22+27=75/5=15 cm^3
then, 15/30= 0.5 cm^3/s(average rate of rxn)
2. 0+15+30+45+60+75+90+105+120=540/9=60 s
0+10+16+22+27+31.50+36+39.5+42=224/9=24.89cm^3
then, 24.89/60=0.414cm^3/s (avrg rate of rxn)
3. overall rxn add all the time divide by 12 and the volume add them too and divide by 12. after take average volume divide by the average time to get the average rate of overall rxn
4. 16/30= 0.533cm^3/s
5. 39.5/105= 0.37cm^3/s
if atom x undergoes radioactive decay and becomes atom y with a half-life of five minutes. after 10 minutes, all number of x atoms have completely turned into to y atoms.
T/F
Answers
True. The fact that atom X undergoes radioactive decay means that it is an unstable atom that will eventually decay into a more stable form, which in this case is atom Y.
The half-life of atom Y is five minutes, which means that it takes five minutes for half of the original amount of atom Y to decay into another substance.
After ten minutes, which is two half-lives of atom Y, all the original number of atom X would have completely turned into atom Y because the first half-life would have transformed half of the atom X into atom Y, and the second half-life would have transformed the remaining half into atom Y. Therefore, the statement is true.
Learn more about radioactive decay here:
brainly.com/question/1770619
#SPJ11
Why would the electrolysis reaction stop if the battery was removed
Answers
If the battery was removed, the energy produced by the battery would not be able to continue its path along the circuit.
Evaluating Lewis Structures
Explain what is wrong with the following structure.
An upper H is single-bonded to a H to the right, which is single bonded to an upper O with pairs of dots above, to the left, and below.
Answers
Answer:
Hydrogen cannot have more than two electrons. Hydrogen bonds to only one other atom at a time.There are too many electrons in the final structure.
Explanation:
Answer:
Explanation:
Hydrogen cannot have more than two electrons. Hydrogen bonds to only one other atom at a time. There are too many electrons in the final structure.
suppose that a new temperature scale has been devised on which the melting point of ethanol (-117.3oc) and the boiling point of ethanol (78.3oc) are taken as 0os and 100os, respectively, where s is the symbol for the new temperature scale. derive an equation relating a reading on this scale to a reading on the celsius scale. what would this thermometer read at 25oc?
Answers
The thermometer would read 40.5°S at 25°C based on new temperature scale.
How to derive the equation for new temperature scale?An equation relating a reading on the new temperature scale (s) to a reading on the Celsius scale can be derived using the two known points of ethanol's melting and boiling points. The freezing point of ethanol is -117.3oc and the boiling point is 78.3oc. So, the equation can be expressed as follows:
S = (100/195.6)*(C + 117.3);
Where S = reading on the new temperature scale;
C => reading on the Celsius scale.
s = (100/195.6)*(25 + 117.3) = 40.5
Therefore, this thermometer would read 40.5°S at 25°C.
It's important to note that this is a hypothetical temperature scale, and it only applies to the freezing and boiling points of ethanol. It doesn't have any practical use and it's not a standard temperature scale like Celsius or Fahrenheit. Also, the freezing and boiling points of ethanol are not constant, they depend on the pressure and the purity of the ethanol.
Learn more about temperature here:
brainly.com/question/18078176
#SPJ4
electrolysis
MgCL(dilute)+H2O=?
Answers
When MgCl2 (Magnesium chloride) dissolves in water gives a faintly acidic solution (pH = approximately 6).
1) How many moles of gas occupy 58 L at a pressure of 1.55 atmospheres and a temperature of 222
K?
Answers
To find the moles of the gas , we can use the ideal gas law. Which states -
\( \:\:\:\:\:\:\:\:\:\star\longrightarrow \sf \underline{PV=nRT} \\\)
Where:-
P is the pressure measured in atmospheres V is the volume measured in litersn is the number of moles.R is the ideal gas constant (0.0821 L atm mol⁻¹ K⁻¹).T is the temperature measured in kelvin.As per question, we are given that-
P=1.55 atmV= 58 LT = 222 KR = 0.08206 L atm mol⁻¹ K⁻¹Now that we have all the required values, so we can put them all in the Ideal gas law formula and solve for moles -
\( \:\:\:\:\:\:\:\:\:\star\longrightarrow \sf \underline{PV=nRT} \\\)
\( \:\:\:\:\:\:\:\:\:\longrightarrow \sf 1.55 \times 58 = n \times 0.0821 \times 222\\\)
\( \:\:\:\:\:\:\:\:\:\longrightarrow \sf 89.9 = n \times 18.2262\\\)
\( \:\:\:\:\:\:\:\:\:\longrightarrow \sf n \times 18.2262 =89.9\\\)
\( \:\:\:\:\:\:\:\:\:\:\:\:\longrightarrow \sf n = \dfrac{89.9}{18.2262}\\\)
\( \:\:\:\:\:\:\:\:\:\:\:\:\longrightarrow \sf n =4.9324......\\\)
\(\:\:\:\:\:\: \:\:\:\:\:\:\longrightarrow \sf \underline{n =4.93 \:moles }\\\)
Therefore, 4.93 moles of gas will be occupied 58 L at a pressure of 1.55 atmospheres and a temperature of 222 k
What caused the color changes in the apple juice titrated without any phenolphthalein present? (Hint: Consider the pigments in apples.) How would you recommend determining the endpoint in the titration of tomato juice?
Answers
The color changes in the apple juice titrated without any phenolphthalein present were caused by the natural pigments present in the apples. By observing the color change of the bromothymol blue, you can accurately determine the endpoint of the titration.
Apples contain a variety of pigments, including chlorophyll, carotenoids, and anthocyanins. As the titration proceeds, the pH of the solution changes, which can cause the pigments to change color.
To determine the endpoint in the titration of tomato juice, I would recommend using a different indicator than phenolphthalein. Tomato juice contains pigments such as lycopene, which can interfere with the color change of phenolphthalein. A better indicator for this titration would be bromothymol blue, which changes from yellow to blue as the pH changes from acidic to basic.
To learn more about Titration :
https://brainly.com/question/186765
#SPJ11
How many atoms of silver metal (Ag) are required to
react completely with 531.8 g of iodine (I2) to produce
silver iodide (AgI)?
Answers
This question is asking for the number of atoms of silver metal that are required to react completely with 531.8 g of iodine to produce silver iodide. At the end, the result turns out to be 2.524x10²⁴ atoms:
What is stoichiometry?In chemistry, stoichiometry is a tool for us to perform mole-mass-particles relationships in chemical reactions. Thus, these problems require a balanced chemical equation, molar masses and the Avogadro's number to proceed.
In such a way. we start by writing the balanced equation for this problem:
\(2Ag+I_2\rightarrow 2AgI\)
Hence, we can calculate the atoms of silver with the following stoichiometric setup:
\(531.8gI_2*\frac{1molI_2}{253.81gI_2}*\frac{2molAg}{1molI_2} *\frac{6.022x10^{23}atomsAg}{1molAg}\)
Where 253.81 is the molar mass of diatomic iodine, 2:1 the mole ratio of silver to iodine and 6.022x10²³ the Avogadro's number. Thus, after solving, we obtain:
\(2.524x10^{24}atomsAg\)
Learn more about stoichiometry: brainly.com/question/9743981
titanium(iv) oxide, tio subscript 2 is brilliantly white and much of the oxide produced is used in the manufacture of paint. what is the maximum amount of tio subscript 2 obtainable from 19.0 tonnes of the ore ilmenite, fetio subscript 3? 10.0 tonnes b 12.7 tonnes c 14.0 tonnes d 17.7 tonnes
Answers
10.0 tonnes of TiO₂ can be produced at most from 19.0 tonnes of the mineral ilmenite, FeTiO₃. The first option is correct.
Utilizing the "mole concept," we may determine the mass of chemical substances according to the situation. A mole is a precise unit of measurement for the number of atoms or molecules in a large sample of the substance. It is the amount of a substance that includes precisely the Avogadro number of the substance's "elementary entities". It is practical to express the quantity of a substance using the mole concept.
For the given situation, first, write the complete equation,
\(\mathrm{FeTiO_3\longrightarrow FeO+TiO_2}\)
Then, the mole concept is introduced as,
\(\begin{aligned}\mathrm{\frac{mole\;of\;TiO_2}{mole\;of\;FeTiO_3}}&=\frac{1}{1}\\\mathrm{mole\;of\;TiO_2}&=\mathrm{mole\;of\;FeTiO_3}\\\mathrm{\frac{m_{TiO_2}}{79.9}}&=\frac{19.0}{151.7}\\\mathrm{m_{TiO_2}}&=\frac{79.9\times19.0}{151.7}\\&=\mathrm{10.00\;tonnes}\end{aligned}\)
The required answer is 10.0 tonnes.
To know more about mole:
https://brainly.com/question/15209553
#SPJ4
rank the following atoms in order of decreasing first ionization energies (i.e., highest to lowest): li, be, ba, f.
Answers
atoms in order of decreasing first ionization energies is given by F>Be>Li>Ba .
What is ionization energy?Ionization energy can be defined simply as a measurement of how difficult it is to remove an electron from an atom or ion or of an atom's or ion's propensity to give up an electron. Usually, when a chemical species is in its ground state, an electron is lost.
As an alternative, we can say that ionisation or ionisation energy is a measurement of the strength of the attractive forces that hold an electron in a specific location.
ionization energy can be defined more precisely as the least amount of energy that an electron in a gaseous atom or ion must absorb to escape the nucleus's influence. It is typically an endothermic reaction and is also known as the ionisation potential.
Ionization is the process of removing an electron from its orbit and moving it outside of the atom. Ionization energy is equal to the difference in energy between the energy of the electron in the initial orbit and the energy of the electron outside the atom since each orbit of the electron has a distinctive energy (in the infinite orbit from the nucleus).
Learn more about ionization energy here :
brainly.com/question/13686323
#SPJ4