Determine the molality of a solution prepared by dissolving 0. 500 moles of caf2 in 11. 5 moles h2o.
Answers
Molality of the given solution is 2.41.
Number of moles of CaF2 = 0.500 Number of moles of H2O = 11.5
Molality is defined as the number of moles of solute per kilogram of solvent.
The formula for molality is given as: Molality = (Number of moles of solute) / (Mass of solvent in kg)
The molecular mass of CaF2 = 40.08 + 2 × 18.99 = 78.06 g/mol
Mass of CaF2 = Number of moles of CaF2 × Molecular
Mass of CaF2 = 0.500 × 78.06 = 39.03 g
Mass of H2O = Number of moles of H2O × Molecular
Mass of H2O = 11.5 × 18.01528 = 207.17 g
Mass of solvent = Mass of H2O = 207.17 g = 0.20717 kg
Molality = (Number of moles of solute) / (Mass of solvent in kg)
Molality = 0.500 / 0.20717
Therefore, the molality of a solution prepared by dissolving 0. 500 moles of caf2 in 11. 5 moles h2o is 2.41.
To know more about Molality visit
https://brainly.com/question/30640726
#SPJ11
Related Questions
what happens to the gaseous phase of water when it reaches a metal lid
Answers
Answer:
When the gaseous phase of water reaches a metal lid it condenses to form water.
Explanation:
The gaseous phase of water is at a higher temperature than the temperature of a metal lid. Thus when it comes in contact with the cold lid it loses heat and gets converted to the liquid phase.
When water vapor loses temperature, it undergoes a process called condensation, which results in its conversion to a liquid state. This process is essentially the opposite of vaporization. Typically, condensation occurs when a vapor is compressed or cooled to its saturation limit, causing the molecular density within the gas phase to reach its maximum threshold.
To know more about condensation,
https://brainly.com/question/30629848
Where do you think the atoms for plant growth come from?
Answers
Answer:
the mass of a tree is primary carbon
Explanation:
the the carbon comes from carbon dioxide during photosynthesis
Answer:
Plants get all the carbon, hydrogen, and oxygen they need from carbon dioxide and water, which they use to build carbohydrates during photosynthesis. To build other kinds of molecules they also need elements like nitrogen, phosphorous, and sulfur. Plants get these as well as other elements from the soil.
Explanation:
explain why the two argon atoms are attracted
to each other.
Answers
- nonmetal and nonmetal equals covalent bond.
A beaker contains 20.0 ml of a 50.0 g/L solution. What will the new concentration be if you add 40.0 mL of water?
Answers
Answer:
moles SO42- = 0.0500 L x 0.20 M=0.010
moles Ba2+ = 0.0500 L x 0.10 M = 0.0050
Ba2+ + SO42- = BaSO4 (s)
moles SO42- in excess = 0.010 - 0.0050=0.0050
total volume = 100 mL = 0.100 L
[SO42-]= 0.0050/0.100= 0.050 M
[Na2SO4] = 2 /2 = 1 M
moles Na2SO4 = 2 M x 0.500 L = 0.500
mass Na2SO4 = 0.500 x 142 g/mol=71.0 g
moles MgBr2 = 46 /184 =0.25
moles Br- = 0.25 x 2 = 0.50
[Br-]= 0.50 / 0.50 L = 1 M
Al + Ni(NO3)2 + Al(NO3)3 + Ni
Answers
Answer:
Aluminum + Nickel(Ii) Nitrate = Nickel + Aluminum Nitrate
Explanation:
What is Angle T?*
S
R
37x-1 23x + 1
т
U

Answers
Explanation:
37x - 1 + 23x + 1 = 180 ° { being co-interior angles }
60x = 180°
x = 180°/ 60
x = 3 °
Now
< T = 37x - 1° = 37 * 3° - 1 = 110°
Hope it will help :)
LINKING IN
TECHNICAL OBJECTS
1 a) What is linking?
Answers
Answer:
A link is a fastening unit that attaches two parts of an object together
Different types of links have different characteristics
Answer:
Linking is the process of combining all the component object files. The linker determines where the code will reside in memory and how control will transfer between the components.
what is the chemical formula for Sulphate...
Answers
Answer:
SO4²
Explanation:
Formula and structure: The sulfate ion formula is SO42- and the molar mass is 96.06 g mol-1. This salt is formed by one sulfate center to which 4 atoms of oxygen are attached, 2 of these atoms are forming S=O.
Answer:
\(so _{4} ^{2 - } \)
Explanation:
sulfate is consisting of sulfur element with 4 oxygen as the structure in the pic given and this group is 2- charged

What would the mystery charge labeled "?" have to be for this object to have a net electric charge of +3?
Answers
For the net charge to be +3,the mystery charge should be is -2 and net force is towards left.
In first problem, overall charge should be -2 (as mentioned in question).So charge labelled with ? should be negative charge ( -ve). There will be net force on -1 charge towards +3 charge (left). For box (b)net force towards right For box c) net force towards right.It is often referred to as electric charge, electrical charge, or electrostatic charge, and denoted by the letter q, is a property of a unit of matter that indicates how many more or less electrons than protons it has.When retained in an electric or magnetic field, matter's basic physical feature known as electric charge causes it to experience a force.To learn more about charge visit:
https://brainly.com/question/14713274
#SPJ9
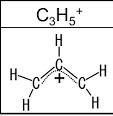
What can you conclude about the symmetry of the C3H5 ion as it actually exists, as regards the bond lengths and the distribution of the positive charge
Answers
In the allyl cation, all the bond lengths are equal and the positive charge is delocalized over all the three carbon atoms.
The allyl cation has the formula C2H5+. In the allyl cation, the positive charge is delocalized over the three carbon atoms as shown in the image attached. Hence, relevant resonance structures can be proposed for the allyl cation.
The bond lengths in the allyl cation are intermediate between the bond lengths of double bonds and single bonds. All the bond lengths are equal.
Learn more: https://brainly.com/question/12108425
Consider a general reaction A(aq)⥫⥬===enzymeB(aq) The Δ°′ of the reaction is −5.540 kJ·mol−1 Calculate the equilibrium constant for the reaction at 25 °C. K′eq= What is Δ for the reaction at body temperature (37.0 °C) if the concentration of A is 1.5 M and the concentration of B is 0.60 M? Δ= Answer needs to be in kJ x mol-1
Answers
a. The equilibrium constant for the reaction at 25 °C from the reaction A(aq) ⥫⥬ enzyme B (aq) and the Δ° of the reaction is -5.540 kJ·mol⁻¹ is 2.98 × 10³.
b. The Δ for the reaction at body temperature (37.0 °C) if the concentration of A is 1.5 M and the concentration of B is 0.60 M is -8.020 kJ·mol⁻¹.
To calculate the equilibrium constant (K'eq) for the reaction at 25°C, we have the relation:
Δ° = -RT ln K'eq
Where R is the universal gas constant, T is the temperature in Kelvin, and ln is the natural logarithm.
Therefore, K'eq = e-Δ°′/RT
Substituting the given values, we have:
K'eq = e-(-5540 J/mol)/(8.314 J/mol K × 298 K)
= 2.98 × 10³
The Δ for the reaction at body temperature (37.0 °C) is given by the relation:
Δ = Δ° + RT ln(Q)
where Q is the reaction quotient at the given concentration of reactants and products.
Q = [B] / [A] = 0.60 / 1.5 = 0.4
Substituting the given values, we have:
Δ = -5540 J/mol + (8.314 J/mol K × 310 K) ln (0.4)
= -8020 J/mol
Therefore, the Δ for the reaction at body temperature is -8.020 kJ·mol⁻¹.
Learn more about equilibrium constant: https://brainly.com/question/29809185
#SPJ11
The dicarboxylic acid, ethanedoic acid, can form a polyester with 1,2-ethanediol. to illustrate the growth of the polymer, draw the trimmer that would form if one ethanedioic acid molecule reacted with two 1,2-ethanediol molecules.
Answers
When ethanedioic acid (HOOC-COOH) reacts with two 1,2-ethanediol molecules (HOCH₂CH₂OH), it forms a trimmer polymer.
What is polymer ?Polymer is a material composed of long chain molecules, or macromolecules, which are made up of many repeating smaller units, known as monomers. Polymer molecules can be natural, such as cellulose, or synthetic, such as plastics and rubbers. Polymers are used to produce a wide range of materials with different characteristics and properties.
The HOOC group of the ethanedioic acid molecule reacts with the two hydroxyl groups of the two 1,2-ethanediol molecules to form the ester linkages. This produces a trimmer polymer, with three monomers connected via two ester linkages.
O
|
HOOC-COO-O-CH₂CH₂-O-CH₂CH₂-OH
|
O
H
To learn more about polymer
https://brainly.com/question/2494725
#SPJ4
how many moles of sodium hydroxide are present in 50.00 ml of 0.09899 m naoh?
Answers
There are approximately 0.00495 moles of sodium hydroxide present in the 50.00 mL solution.
To find the moles of sodium hydroxide (NaOH) in a 50.00 mL solution with a concentration of 0.09899 M, you can use the formula:
moles = volume (L) × concentration (M)
First, convert the volume from mL to L:
50.00 mL = 0.05000 L
Now, multiply the volume in liters by the concentration:
moles = 0.05000 L × 0.09899 M
moles ≈ 0.00495 mol
Therefore, there are approximately 0.00495 moles of sodium hydroxide present in the 50.00 mL solution.
To learn more about volume, refer below:
https://brainly.com/question/1578538
#SPJ11
Humans rely on water for
Answers
Answer:
almost everything
Explanation:
How can you determine an atom's atomic mass?
Answers
Answer: I think you're supposed to add the protons and neutrons and then that's your answer. Sorry if I'm wrong :/
Explanation:
A 9400. 0 mL container holds a sample of nitrogen gas at 15. 00C and 93. 0inHg. Determine the number of moles of nitrogen gas in the cylinder
Answers
The number of moles of nitrogen gas in the cylinder is calculated by dividing the product of pressure, volume, and temperature by the ideal gas constant.
To determine the number of moles of nitrogen gas in the cylinder, we can use the ideal gas law equation:
PV = nRT
Where:
P = pressure in atmospheres
V = volume in liters
n = number of moles
R = ideal gas constant (0.0821 L·atm/(mol·K))
T = temperature in Kelvin
First, we need to convert the given pressure from inches of mercury (inHg) to atmospheres (atm). Using the conversion factor 1 inHg = 0.0334211 atm, we find:
P = 93.0 inHg * 0.0334211 atm/inHg = 3.10877 atm
Next, we need to convert the given temperature from Celsius to Kelvin. Adding 273.15 to the Celsius temperature, we have:
T = 15.00°C + 273.15 = 288.15 K
Now we can rearrange the ideal gas law equation to solve for the number of moles (n):
n = PV / RT
n = (3.10877 atm) * (9.400 L) / (0.0821 L·atm/(mol·K) * 288.15 K)
Calculating this expression will give us the number of moles of nitrogen gas in the cylinder.
Learn more about moles of nitrogen gas in the cylinder here:
https://brainly.com/question/31598827
#SPJ11
The activation of the serine/threonine protein kinase akt requires phosphoinositide 3-kinase (pi 3-kinase) to:______.
Answers
The activation of the serine/threonine protein kinase akt requires phosphoinositide 3-kinase (pi 3-kinase) to create phosphorylated lipids that serve as docking sites that localize Akt to the plasma membrane.
Ak strain changing is the meaning of the word Akt. The term "Akt" first appeared in 1928, when J. Furth conducted research on mice that spontaneously generated thymic lymphomas.
An essential enzyme in the signaling pathway that controls how cells react to insulin as well as other growth stimuli is called phosphoinositide 3-kinase (PI3K). To create phosphatidyl-inositol-3,4,5-trisphosphate (PIP3) at the plasma membrane, one such enzyme phosphorylates phosphatidylinositol-4,5-bisphosphate in the third carbon in the molecule.
Therefore, The activation of the serine/threonine protein kinase akt requires phosphoinositide 3-kinase (pi 3-kinase) to create phosphorylated lipids that serve as docking sites that localize Akt to the plasma membrane.
To know more about protein kinase akt
https://brainly.com/question/17092864
#SPJ4
rank the ions in each set in order of increasing size. a. li , k , na b. se2– , rb , br – c. o2– , f – , n3–
Answers
The correct order of increasing size is in each set is: Li⁺ < Na⁺ < K⁺, Br⁻ < Se²⁻ < Rb⁺, and N³⁻ < O²⁻ < F⁻.
a. In order of increasing size, the ions in set a are: Li, Na, K. This is because they all have the same charge (+1), but as you move down the periodic table, the atomic radius increases.
b. In order of increasing size, the ions in set b are: Br-, Se2-, Rb. This is because Br- and Se2- have the same charge (-1), but as you move down the periodic table, the atomic radius increases. Rb has a larger atomic radius than Se, which gives it a larger ionic radius.
c. In order of increasing size, the ions in set c are: N3-, O2-, F-. This is because they all have the same charge (-1), but as you move across the periodic table, the atomic radius decreases. F- has the smallest atomic radius, which gives it the smallest ionic radius.
Know more about Atomic Radius here:
https://brainly.com/question/14086621
#SPJ11
need help, no idea what to do

Answers
The volume of unreacted Oxygen = 1.25 L = 1.25 dm³ = 1250 cm³
Further explanationGiven
Combustion of propane
3.6 L CO2
7.25 L O2
Required
The volume of unreacted oxygen
Solution
Reaction
C₃H₈ + 5O₂ ⇒ 3CO₂ + 4H₂O
Avogadro's :
In the same T,P and V, the gas contains the same number of molecules
So the ratio of gas volume will be equal to the ratio of gas moles
Vol O₂ = 5/3 x vol CO2 = 5/3 x 3.6 = 6 L
The unreacted : 7.25 - 6 = 1.25 L
How does the type of medium affect a sound wave?
Answers
sound waves travel faster in solids than in liquids, and faster in liquids than in gasses. While the density of a medium also affects the speed of sound, the elastic properties have a greater influence on the wave speed.
hope this helped
What defines an element?
Answers
element is made up of only one type of atoms.
it is pure form of matter
example hydrogen, oxygen, carbon etc
Answer:
An element is a substance that is made up of only one kind of atom and cannot be broken down into simpler forms by any ordinary chemical means
Enzymes are able to act on any molecules they come in contact with . True or false
Answers
Answer:
Explanation:
True
If 50.0g of nitrogen gas occupies a volume of 85L at a given temperature and pressure, what volume will 45.0g of nitrogen occupy at the same temperature and pressure?
Answers
Explanation:
You want 45 out of the 50's ....or 45 / 50ths of the 85 L
45/50 * 85 = 76.5 L
The atomic number of magnesium is 12.Find the number of electrons , protons and neutrons?
Answers
Answer: there are 12 electrons 12 protons and 12 neutrons.
i hope this helps.
Which of the following is an amorphous solid?
O
A. Diamond
B. Graphite
O C. Glass
O D. Iron
Answers
Answer:
C. Glass
Explanation:
Amorphous solids have a non-crystalline structure and no order. In that case, Diamonds, Graphite, and Iron all have a crystalline structure and order. You are left with C as your answer.
There are moles of carbon present in 100 g of a
compound with the following percentage composition:
C= 40.0%, H = 6.72%, O = 53.29%.
Answers
Answer: 3.33
Explanation:
The molecular formula of the given composition is C₃H₆O₃.
What is molecular formula?Molecular formula is defined as a compound's chemical formula, which counts the total number of each element present in a molecule of the compound as discrete molecules. The arrangement of atoms in a molecule in three dimensions is known as molecular geometry, commonly referred to as the molecular structure.
The percentage composition is
C = 40.00 %
H = 6.72 %
O = 53.29 %
Mole = 100 g
Mass of C = 40 / 100 x 100 = 40 g
mass of H = 6.72 / 100 x 100 = 6.72 g
Mass of O = 53.29 / 100 x 100 = 53.29 g
Dividing the mass with moles
C = 40 / 12 = 3.3
H = 6.72 / 1 = 6.72
O = 53.29 / 16 = 3.3
Formula = C₃H₆O₃
Thus, the molecular formula of the given composition is C₃H₆O₃.
To learn more about molecular formula, refer to the link below:
https://brainly.com/question/28647690
#SPJ2
What properties of a soccer ball would change when it is filled with air particles?
Color and weight
Length and width
Size and shape
Texture and mass
Answers
What three types of Protists have been discovered
a. Animal-like, fungus-like, insect-like
b. Animal-like, mammal-like, fungus-like
c. Animal-like, single-celled, plant-like
d. Animal-like, fungus-like, plant-like
Answers
Answer:
the answer is D
Explanation:
but hope this helps
Answer:
Yo, wassup I think its D.
if 140cm3 of methane diffuse into air in 72 sec,how long will it take 210cm3 of sulphur dioxide to diffuse under the same condition ?
Answers
Answer:
time for sulphur dioxide to diffuse is 221.05 secs
The time for sulphur dioxide to diffuse is 108 sec.
Which gas diffuses twice quickly as SO2?Sulfur dioxide has a molecular weight of sixty-four and as a result, the square root is 8. since its inversely proportional, on the way to diffuse two times as fast, the rectangular root of the molecular weight should be 4, and subsequently the molecular weight might be 16. So the solution is methane (CH4).
Which gases diffuse the fastest?The charge of diffusion for a gas is inversely proportional to the rectangular root of its molecular mass (Graham's law). The gasoline with the lowest molecular weight will effuse the fastest. The lightest, and consequently fastest, fuel is helium.
Learn more about Sulfur dioxide here: https://brainly.com/question/15654465
#SPJ2
Balance the following equation: B2O3(s) + HF(l) BF3(g) + H2O(l) A. B2O3(s) + 6HF(I) -> 2BF3(g) + 3H2O(l) B. B2O3(s) + H6F6(l) -> B2F6(g) + H6O3(l) C.B2O3(s) + 2HF(l) -> 2BF3(g) + H2O(l) D. B2O3(s) + 3HF(l) -> 2BF3(g) + 3H2O(1) E. B2O3(s) + 6HF(l) -> 2BF3(g) + 6H2O(1)
Answers
B2O3 (s) + 6HF (l) ---------> 2BF3 (g) + 3 H2O (l) is the balanced equation.
A chemical equation is a graphical representation of the a chemical reaction using symbols but also chemical formulas. The reactant enterprises are on the left, and the product entities are on the right, with a plus sign between both the entities both the reactants and products, as well as an arrow pointing towards the products to indicate the reaction's direction.
Given reaction is
B2O3 (s) + HF (l) ---------> BF3 (g) + H2O (l)
On left side 2 B atoms. To equalize B atoms , put coefficient 2 for BF3.
B2O3 (s) + HF (l) ---------> 2BF3 (g) + H2O (l)
Now on right side 6 F atoms. To equalize F atoms , put coefficient 6 for HF.
B2O3 (s) + 6HF (l) ---------> 2BF3 (g) + H2O (l)
Now on right side 6 H atoms. To equalize H atoms , put coefficient 3 for H2O.
B2O3 (s) + 6HF (l) ---------> 2BF3 (g) + 3 H2O (l)
Now all atoms are balanced.
Therefore, balanced equation is B2O3 (s) + 6HF (l) ---------> 2BF3 (g) + 3 H2O (l)
To know more about the Balanced Equation, here
https://brainly.com/question/12192253
#SPJ4