Constitutional Isomerism
Problem: Draw structural formulas for the five constitutional isomers with the molecular formula C6H14.
Answers
Constitutional isomerism is a type of isomerism where molecules have the same molecular formula but differ in the way their atoms are arranged.
In other words, they have different structural formulas. For the molecular formula C6H14, there are five constitutional isomers possible. Here are their structural formulas:
1. Hexane - CH3CH2CH2CH2CH2CH3
2. 2-Methylpentane - CH3CH2CH(CH3)CH2CH3
3. 3-Methylpentane - CH3CH2CH2CH(CH3)CH3
4. 2,2-Dimethylbutane - (CH3)3CCH2CH3
5. 2,3-Dimethylbutane - CH3CH(CH3)CH(CH3)CH3
These five constitutional isomers have the same molecular formula C6H14 but different structural formulas, which gives them different physical and chemical properties.
To know more about Constitutional isomerism refer
https://brainly.com/question/31497008
#SPJ11
Related Questions
What is the concentration of H+ at pH 4 in moldm-3 ?
Answers
Answer:
The pH of a solution is a measure of its concentration of hydrogen ions. The higher the concentration of H + ions in an acidic solution, the lower the pH. A pH of 1 represents a hydrogen ion concentration of 0.1 mol/dm 3.
...
pH and hydrogen ion concentration.
Concentration pH
0.001 mol/dm 3 3
0.0001 mol/dm 3 4
Explanation:
Answer:
0.0001
That the answer your welcom
When a forensic analyst determines the chemical composition of a preparation that may contain illicit drugs such as heroin, cocaine, or barbiturates, this is an example of what?.
Answers
this is an example of Identification
Technicians in forensic science work at crime scenes and in labs. Typically, forensic science technicians do the following tasks at crime scenes: Investigate crime scenes to decide what evidence needs to be gathered and how. The crime scene and the evidence should both be photographed. In forensic crime labs, forensic DNA analysts perform analyses on samples collected from crime scenes. While some forensic DNA analyzers work for privately owned forensic laboratories, the majority of them are employed by local, state, or federal law enforcement or governmental organizations. The definition of forensic analysis is a thorough procedure for identifying, looking into, and recording the cause, progression, and effects of a security incident or violation of local, state, and organizational regulations.
To know more about forensic analyst refer to https://brainly.com/question/23528771
#SPJ4
please help!2008下
2. (20) The following gaseous reaction is used for the manufacture of 'synthesis gas': CH4 + H₂O
Answers
The gaseous reaction used for the manufacture of 'synthesis gas' is CH4 + H2O.
The reaction CH4 + H2O is a chemical reaction that involves the combination of methane (CH4) and water (H2O) to produce synthesis gas. Synthesis gas, also known as syngas, is a mixture of carbon monoxide (CO) and hydrogen gas (H2). It is an important intermediate in various industrial processes, including the production of fuels and chemicals.
In this reaction, methane (CH4) and water (H2O) react in the presence of suitable catalysts and/or high temperatures to form synthesis gas. The reaction can be represented by the equation:
CH4 + H2O → CO + 3H2
The methane and water molecules undergo a chemical transformation, resulting in the formation of carbon monoxide (CO) and hydrogen gas (H2). The synthesis gas produced can be further processed and utilized for various purposes, such as the production of methanol, ammonia, or hydrogen fuel.
The reaction CH4 + H2O is used in the manufacture of synthesis gas. This reaction involves the combination of methane and water to produce carbon monoxide and hydrogen gas. Synthesis gas is an important intermediate in industrial processes and finds applications in the production of fuels and chemicals.
To learn more about synthesis gas, visit
brainly.com/question/13873835
#SPJ11
Na2CO3 is a/an ____________ compound where its electrons are _______. *
1.covalent, shared
2.covalent, gained/lost
3.ionic, gained/lost
4.ionic, shared
Answers
Answer:
Answer is 3 because NaCo3 because NaCo3 is ionic compound and electrons of ionic compound are gained / lost
If 10 moles of P4S3 was used, how many grams of P4O6 was produced? Leave up to 3 decimal places when possible.
Answers
If 10 moles of P4S3 were used, the mass of P4O6 produced would be 2838.8 grams.
To determine the number of grams of P4O6 produced from 10 moles of P4S3, we need to use the balanced chemical equation and the molar masses of the compounds involved.The balanced equation for the reaction between P4S3 and oxygen to produce P4O6 is:
P4S3 + 8 O2 → P4O6 + 6 SO2
From the balanced equation, we can see that the molar ratio between P4S3 and P4O6 is 1:1. This means that for every 1 mole of P4S3 consumed, 1 mole of P4O6 is produced.The molar mass of P4S3 is 220.25 g/mol, and the molar mass of P4O6 is 283.88 g/mol.
To calculate the mass of P4O6 produced, we can use the following equation:
Mass of P4O6 = Moles of P4O6 × Molar mass of P4O6
Since the molar ratio between P4S3 and P4O6 is 1:1, the number of moles of P4O6 produced is also 10 moles.
Mass of P4O6 = 10 moles × 283.88 g/mol = 2838.8 grams
for such more questions on mass
https://brainly.com/question/24191825
#SPJ8
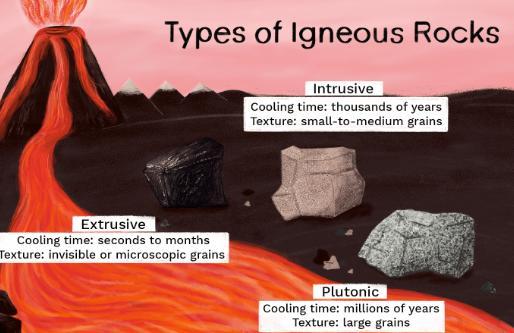
Create a model using images that would show what would happen to the igneous rock when it is exposed to different energy sources.
Answers
igneous is moved to Earth's surface and exposed to energy from the sun, it could weather into smaller rock pieces that could form sedimentary rock

Identify the following as an element or compound. sodium metal (Na) element compound
Answers
sodium (Na), chemical element of the alkali metal group..
Sodium is a very soft silvery-white metal. Sodium is the most common alkali metal and the sixth most abundant element on Earth, comprising 2.8 percent of Earth's crust.
#Cutest Ghoststrong acids and bases completely dissociate in water. use the table in the introduction to classify the following chemical compounds as strong acids, weak acids, strong bases, and weak bases.
Answers
To classify the chemical compounds as strong acids, weak acids, strong bases, and weak bases, I would need the table you mentioned in the introduction.
Strong acids are those that completely dissociate in water, meaning they release all of their hydrogen ions (H+) when dissolved. Some common examples include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
Weak acids do not completely dissociate in water and only release a small fraction of their hydrogen ions. Examples include acetic acid (CH3COOH), phosphoric acid (H3PO4), and hydrofluoric acid (HF).
Strong bases completely dissociate in water, releasing hydroxide ions (OH-). Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
Weak bases, like weak acids, do not completely dissociate in water. They react with water to form a small number of hydroxide ions. Examples include ammonia (NH3), methylamine (CH3NH2), and pyridine (C5H5N).
Please provide the specific chemical compounds and the table for a more accurate classification.
To know more about chemical compounds visit:
https://brainly.com/question/12166462
#SPJ11
A central atom has two lone pairs on opposite sides and four single bonds. What is the molecule geometry of the result?
Answers
The molecular geometry is square planar.
According to the Valence Shell Electron Pair Repulsion theory (VSEPR), the shape of a molecule is determined by the number of valence electrons surrounding the outermost shell of the central atom in the molecule.
In this case, the expected geometry based on VSEPR theory is octahedral. However, the lone pairs on opposite sides of the four single bonds leads to a square planar molecular geometry.
Learn more; https://brainly.com/question/24396703
1. Which of the following is NOT a main tissue type? *
Answers
Answer:
there is nothing follow that
Explanation:
what happens when chloro Methane is heated with sodium in the presence of dry ether ?(wurtz reaction)
Answers
The Wurtz reaction occurs when chloromethane is heated with sodium in the presence of dry ether. The reaction produces two molecules of methane and sodium chloride.
Why is dry ether needed to make the Grignard reagent?Since it forms a stable complex when reacting with Grignard reagents, ether is also utilised as a solvent. The ionic magnesium-halogen link dissolves the ether's carbon-oxygen bond, creating a stable complex and enhancing the Grignard reagent's reactivity.
When dry ether is present, what is treated with magnesium?Find out how Grignard's reagent affects carbonyl compounds. Based on this, you may use the reaction mechanisms for the specified named reactions to identify the first responding molecule.
To know more about Wurtz reaction visit:-
https://brainly.com/question/28330368
#SPJ1
What is the pOH of water?

Answers
Answer:
A. 7
(assuming the water is neutral)
Determine the number of protons, neutrons, and electrons in the isotopes below

Answers
Sr-38 protons, 38 electrons, 50 neutrons
Li-3 protons, 3 electrons, 4 neutrons
Bi-83 protons, 83 electrons, 126 neutrons
Reply to this if you want me to explain why:)
MOST of the elements in the periodic table are
A. liquids at room temperature
B. metals
C. metalloids
D. nonmetals
Answers
Answer:
B. metals
Explanation:
Most of the elements in the periodic table are classified as metals. They are usually solid at room temperature.
The first and second block of the periodic table are all metallic elements. Those in the d-block are all metals. Metals generally occupy the s and d -blocks. Non-metals and metalloids are found in the p-blocksAs the moon orbits the ______________, its gravitational pull is______________ on the side of the earth closest to the ______________.This ______________ force pulls on the water facing the moon,creating a ______________. The moon also ______________ on the solidearth, causing the water on the far side of earth to ______________as well. These bulges in the water are the ______________.The areas in between the close and far side of the earth which are not in ______________ with the moon experience ______________.
pls help i give brainlyest
Answers
Answer:
Earth
Strongest
Moon
Gravitational
Tide
Pulls
Bulge
Waves
Proximity
Low Tide
Which geologic features help scientists determine the relative ages of rocks by their positions? Select three options.
magma
erosion
intrusions
index fossils
cross-cutting relationships
helppp i need it nowwwww
Answers
Answer:
Option D, Index fossil helps scientists to determine the relative ages of rocks by their positions
Explanation:
Index fossils are arranged in layer in such a way that the lowest layer represent the oldest fossil and the top most layer represents the youngest fossil. Scientist use this concept to determine the relative age of the rocks based on their position beneath the earth’s surface
Hence, option D is correct
PLEASE HELP ME 40 POINTS RIGHT ANSWERS ONLY!!!!! :)
Consider the solubility curve at right. which solid material is a solid solute?

Answers
Substance C is a solid solute because the solubility of a solid increases with increasing temperature. Therefore, option B is correct.
Solubility refers to the ability of a solute to dissolve in a solvent and form a homogeneous mixture called a solution. It is a measure of how much of a solute can dissolve in a given amount of solvent under specific conditions, such as temperature and pressure.
Solubility is typically expressed as the maximum amount of solute that can dissolve in a specified amount of solvent. The solubility of a substance is influenced by various factors, including the nature of the solute and solvent, temperature, pressure, and the presence of other substances.
Learn more about solubility, here:
https://brainly.com/question/31493083
#SPJ1
Answer: it's substance A hope it helps.!
find the co valance of c in C2S2
Answers
Answer:
A better term than
covalency
might be
molecularity
. Molecular species TEND to have reduced melting points and boiling points with respect to non-molecular species. Non molecular species, which include sodium chloride, and graphite, and diamond, thus have much HIGHER melting points than methane or ethane, or water, which are composed of discrete molecules.
Explanation:
Which organism is a producer? A:owl B:mouse C:grass D:snake please somebody help me
Answers
Answer:
I think it's C. Grass. :)
what is the number of oxyegen on periodic table?
Answers
Answer:
16
Explanation:
Answer:
Oxygen has an atomic number of 8
Explanation:
the following experiment was carried out using a newly synthesized chlorofluorocarbon. exactly 50 ml of the gas effused through a porous barrier in 157 s. the same volume of argon effused in 76 s under the same conditions. which compound is the chlorofluorocarbon?
Answers
The molar mass corresponds to the chlorofluorocarbon CF3Cl (Freon-11), which has a molar mass of 137.37 g/mol. Therefore, the chlorofluorocarbon in the experiment is CF3Cl.
The rate of effusion of a gas through a porous barrier is inversely proportional to the square root of its molar mass. Therefore, we can use the rate of effusion to determine the relative molar mass of the two gases and identify which one is a chlorofluorocarbon.
The rate of effusion can be calculated using Graham's law:
Rate of effusion = Volume of gas / Time taken to effuse
For the chlorofluorocarbon, the rate of effusion is:
Rate of effusion (CFC) = 50 mL / 157 s = 0.3185 mL/s
For argon, the rate of effusion is:
Rate of effusion (Ar) = 50 mL / 76 s = 0.6579 mL/s
Using Graham's law, we can set up the following equation:
Rate of effusion (CFC) / Rate of effusion (Ar) = sqrt(Molar mass (Ar) / Molar mass (CFC))
Solving for the ratio of molar masses:
Molar mass (Ar) / Molar mass (CFC) = (Rate of effusion (Ar) / Rate of effusion (CFC))^2
Molar mass (Ar) / Molar mass (CFC) = (0.6579 mL/s / 0.3185 mL/s)^2
Molar mass (Ar) / Molar mass (CFC) = 4.294
Molar mass (CFC) = Molar mass (Ar) / 4.294
The molar mass of argon is 39.95 g/mol. Therefore, the molar mass of chlorofluorocarbon is:
Molar mass (CFC) = 39.95 g/mol / 4.294 = 9.30 g/mol
To learn more about molar mass
https://brainly.com/question/13152455
#SPJ4
Zinc chloride + Magnesium →
Answers
Answer:
im assuming you need the balanced equation
Explanation:
Zn + MgCl2 = ZnCl2 + Mg
Answer:
it will mgcl2 + zn
Explanation:
Mg will replace zn from its salt solution
to form mgcl2
hope it helps
What is an ion? Write its types with example.
Answers
Answer:
an ion is an element that carries either positive or negative charge. the example are cation which has a positive charge e.g H+ and anion which had a negative charge eg Cl-.
An ion is a positively or negatively charged atom (or group of atoms). An ion is formed by the loss or gain of electrons by an atom, so it contains an unequal number of electrons and protons. Example: Sodium ion Na+, magnesium ion Mg2+, chloride ion Cl–, and oxide ion O2–.
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
Calculate the pressure exerted by 200. g of A r in a rigid 4.50 L container at 21.0 ˚ C . Assume ideal gas behavior. Note that R = 0.08206 L ⋅ atm K ⋅ mol .
Answers
The pressure exerted by 200 g of Ar in a rigid 4.50 L container at 21.0 ˚ C would be 19.6 atm.
Ideal gas problemTo calculate the pressure exerted by the Argon gas, we can use the ideal gas law:
PV = nRT
where
P is the pressureV is the volumen is the number of molesR is the ideal gas constantT is the temperature in Kelvin.First, we need to determine the number of moles of Argon gas present:
n = mass / molar massn = 200/39.95 = 5.004 molesNext, we convert the volume and temperature:
V = 4.50 L = 0.00450 \(m^3\)T = 21.0 ˚C + 273.15 = 294.15 KNow we can substitute the values into the ideal gas law and solve for P:
P = nRT/VP = (5.004) x (0.08206) x (294.15) / (0.00450)P = 19.6 atmIn other words, the pressure exerted by 200 g of Argon gas in a 4.50 L container at 21.0 ˚C is 19.6 atm.
More on ideal gas can be found here: https://brainly.com/question/31463642
#SPJ1
which countries cannot be used to distinguish physical characteristics of metals and non-metals?
A) brittle
B) Malleability
C) Reactivatity
D) Shiny Luster
Answers
Answer: A
Explanation: I got it right on mine
Which of the following best describes how Thomson
concluded that electrons are present in all atoms.
a
The charge-to-mass ratio of electrons is always the
same constant, no matter which substances are
used in the cathode ray tube.
b The cathode ray was able to turn a wheel.
The cathode ray always bent toward a negatively
charged plate.
d
The charge-to-mass ratio is unique to each different
element.
Answers
Answer: A
Explanation:
The charge-to-mass ratio of electrons is always the same constant, no matter which substances are used in the cathode ray tube.
Explanation:
Thomson in his model of atom discussed that the atom consists of a negative charge particle termed electron randomly distributed in the positively charged sphere to balance the negative charge.His model of the atom was also known as the Plum pudding model of the atom. In which electrons are embedded in positive soup.He discovered electrons by conducting an experiment with cathode rays in which cathode rays emerging from the cathode were observed to be deflected towards the positively charged plate.He also conducted the same experiment with different metals (for anode and cathode) and gases and found out that the charge to mass ratio of the electron was regardless of the metals or gases used in the experiment.With this, he landed on the conclusion that these particles of cathode rays are the universal component of matter.So, from this, we can conclude that the charge-to-mass ratio of electrons is always the same constant, no matter which substances are used in the cathode ray tube describes Thomson's conclusion that electrons are present in all atoms.
Learn more about J.J. Thomson's model of atom here:
brainly.com/question/2437167?referrer=searchResults
brainly.com/question/1874920?referrer=searchResults
Which shows an isomer of the molecule below?

Answers
Answer: C
Explanation:
Don’t trust the other person it’s not A
*view photo for answer*
pay attention! the answer choices aren't always in the same order!

Define kinetic energy. A) energy associated with the temperature of an object B) energy associated with the motion of an object C) energy associated with the force of an object D) energy associated with the gravity of an object E) energy associated with the position or composition of an object 2) Determine the density of an object that has a mass of 1498 g and displaces 12.1 mL of water when placed in a graduated cylinder. A) 8.08 g/mL B) 1.38 g/mL C) 12.4 g/mL D) 18.1 g/mL E) 11.4 g/mL 3) How many significant figures are in the measurement, 20.300 m?! A)3 B) 4 C) 5 D) 1 E)2 4) What does "X" represent in the following symbol? 80 358 A) mercury B) chlorine C) scandium D) bromine E) selenium 5) Write the formula for copper (II) sulfate pentahydrate. A) Cu2SO3 H5 B) Cu2S'H20 C) CuS 5H20 D) (CuSO4)5 E) CuSO4'5H20
Answers
1. B, the energy associated with the motion of an object
2. C, 12.4 g/mL
3. C, 5
1) B) Kinetic energy is energy associated with the motion of an object.
2) B) Density equals mass divided by volume: 1.38 g/mL
3) C) There are 5 significant figures in 20.300
4) A) 80 is the atomic number for mercury on the periodic table.
5) E) The formula for copper (II) sulfate pentahydrate is CuSO4•5H2O
So in summary:
• Kinetic energy is the energy of a moving object due to its motion.
• Density is calculated by dividing an object's mass by its volume.
• Significant figures refer to the known precision of a measurement based on the digits reported.
• Atomic symbols represent elements on the periodic table.
• Chemical formulas use symbols of the elements to show the proportions of atoms in a compound.
Consider a sample of gas with 6.022 x 10^23 molecules. At a temperature of 25.0 C and a pressure of 125 kPa what would be the volume ?
Answers
The volume of the gas at a temperature of 25°C is equal to 19.82 L.
What is the ideal gas equation?The ideal gas law can be defined as the general equation to describe the state of a perfect gas. This equation represents the product of the volume and pressure is equal to the product of the gas constant, number of moles of gas, and absolute temperature.
The ideal gas equation for a gas can be represented as shown below:
PV = nRT
where n is the moles of gas, T is the temperature, P is the pressure, V is the volume, and R is the gas constant.
Given, the number of molecules of gas = 6.022 × 10²³
The number of moles of gas = 1mol
The temperature of gas, T = 25°C = 25 + 273.15 = 298.15 K,
The pressure of gas, P = 125 kPa = 1.233 atm
Gas constant, R= 0.082 atmL/K mol
Substituting the values n, R, P, and T in the equation, we get:
1.233 × V = 1 × 0.082 × 298.15
V = 19.82 L
Learn more about ideal gas equation, here:
brainly.com/question/3637553
#SPJ1