The generation of a magnetic field by an electric current is
Answers
Answer:
The generation of a magnetic field by an electric current is called electromagnetism.
Explanation:
Answer:
The generation of a magnetic field by an electric current is called electromagnetism.
Related Questions
4.Calculate the volume of 40g of Helium (He) at rtp.
Answers
Answer:
2.24dm³
Explanation:
Given parameters:
Mass of He = 40g
Unknown:
Volume of Helium = ?
Solution:
To solve this problem, we convert the given mass to number of moles.
Number of moles = \(\frac{mass}{molar mass}\)
molar mass of He = 4g/mol
Number of moles = \(\frac{4}{40}\) = 0.1mole
So;
1 mole of gas at rtp occupies a volume of 22.4dm³
0.1 mole of He will occupy a volume of 0.1 x 22.4 = 2.24dm³
Match groups with their appropriate electron configuration
Group 15
Group 18
Lathanides and Actinides
Group 17
Group 1
Group 14
Group 16
Groups 3-12
Group 2
Group 13
a. s2 p4
b. s1
c. s2 p6
d. s2 p2
e. s2 p1
f. s2 p5
9. s2
h. fill d orbitals
i. fill f orbitals
j. s2 p3
Answers
The valence electronic configuration of group 17 is s² p⁵, and that for group one is s¹ and group 16 is s² p⁴. For group 3 -12 the valence electrons fill in d - orbitals. and for group 2 it is s². for group 13- s² p¹. For lanthanides and actinides, the valence electrons fill in f- orbitals.
What is electronic configuration?The filling of electrons of an atom from the lower energy level to the higher energy level is called its electronic configuration. The group number for atom with 3 and more electrons are 10 added to these numbers.
For group 15, there are 5 valence electrons and the valence electronic configuration is : s² p³.
For group 18 : s² p⁶.
For group 16 : the valence configuration is s² p⁴. And for group 17 it is s² p⁵. Group 1 and 2 have the valence electronic configuration as s¹ and s² respectively.
Group 14, the configuration is s² p² and the that for group 13 is s² p¹. Elements of group 3 - 12 are d - block elements. The valence electrons of these elements fall in d- orbital.
Similarly lanthanides and actinides are f - block elements. Their valence electrons are filled in f- orbitals.
To find more on electronic configuration, refer here:
https://brainly.com/question/29757010
#SPJ1
Match each chemical reaction with the type of reaction that best describes it
Answers
Explanation:
Types of Chemical ReactionCombination Reaction.Decomposition Reaction.Displacement Reaction.Double Displacement Reaction.Oxidation and Reduction Reaction.stay safe healthy and happy.40 POINTS! Will Mark Brainliest if correct answer and all work is shown!
In this reaction: Mg (s) + I₂ (s) → MgI₂ (s)
If 2.08 moles of Mg react with 3.56 moles of I₂, and 1.76 moles of MgI₂ form, what is the percent yield?
Answers
Explanation:
here's the answer to your question

Answer:
I'm not really sure. Sorry dude.
Explanation:
Sorry.
a hypoeutectoid steel is one with an alloy composition between that of the left-hand end of the tie line defining the eutectoid reaction and the eutectoid composition, i.e., between ---select--- weight percent carbon. it is common though to refer to any composition to the ---select--- of the eutectoid point as hypoeutectoid. a hypereutectoid steel is one with an alloy composition between ---select--- wei
Answers
A hypoeutectoid steel is one with an alloy composition between that of the left-hand end of the tie line defining the eutectoid reaction and the eutectoid composition, i.e., between 0 and 0.76 weight percent carbon. It is common though to refer to any composition to the left of the eutectoid point as hypoeutectoid. A hypereutectoid steel is one with an alloy composition between 0.76 and 2.14 weight percent carbon.
In simpler terms, hypoeutectoid steel contains less carbon than the eutectoid composition (0.76 weight percent carbon), while hypereutectoid steel contains more carbon than the eutectoid composition. The eutectoid point is where the steel has the perfect balance of carbon content and can exist in both austenite and ferrite phases at a specific temperature (the eutectoid temperature).
When cooling a hypoeutectoid steel, it first forms a proeutectoid ferrite phase, followed by the eutectoid transformation of the remaining austenite into pearlite (a mixture of ferrite and cementite). This results in a microstructure with ferrite and pearlite phases.
On the other hand, cooling a hypereutectoid steel leads to the formation of proeutectoid cementite, followed by the eutectoid transformation of the remaining austenite into pearlite. This results in a microstructure with cementite and pearlite phases.
Understanding the differences between hypoeutectoid and hypereutectoid steels is important in selecting the appropriate material for specific applications, as their mechanical properties, such as strength and ductility, can vary significantly.
for more such question on hypoeutectoid steel
https://brainly.com/question/27433512
#SPJ11
How many oxygen atoms are in the products of the balanced reaction below?
3Ca + 2AIPO4 + 2Al + Ca3(PO4)2
A. 12
B. 8
C. 3
D. 4
Answers
Answer:
B
Explanation:
production ca3(PO4)2
4x2=8
A beaker is filled with water to the rim. Gently placing a plastic toy duck in the beaker causes some of the water to spill out. The weight of the beaker with the duck floating in it is
Answers
The weight of the beaker with the duck floating in it will be the weight of the beaker plus the weight of the water that was displaced by the duck, which is equal to the weight of the duck.
The weight of the beaker with the duck floating in it will be the same as the weight of the beaker with water before the duck was added, plus the weight of the duck itself.
Assuming that the volume of the duck is negligible compared to the volume of the water in the beaker, the weight of the displaced water (the water that spills out when the duck is added) will be equal to the weight of the duck.
This is known as Archimedes' principle, which states that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid displaced by the object.
In other words, the weight of the beaker with the duck floating in it will be the weight of the beaker plus the weight of the duck.
For more question on weight click on
https://brainly.com/question/28571689
#SPJ11
why does the existence of unpaired electrons in the valence shell determine the reactivity of an atom
Answers
The electrons that are found in an atom's outermost shell are known as valence electrons. The reason for this is because if atoms engage, the electrons in their outermost shells are the first to make contact with one
Which electrons have valence?The electrons located in an atom's valence shell, and energy level, are known as valence electrons. For instance, oxygen contains two valence electrons in the 2s subshell as four with in 2p subshell, for a total of six valence electrons. The oxygen's valence electron configuration is 2s22p4.
Are electron shells always eight?The octet rule, a general rule of thumb, states that most biologically significant elements require eight electron in the outer shell to be stable. Even if the valence shell is the some shell, that has a maximum capacity for holding 18 electrons, some atoms can nevertheless be stable with an octet.
To know more about valence electrons visit:
https://brainly.com/question/13993867
#SPJ4
What's IUPAC ?
[●] _/_\_ [●]
Answers
Answer:
International Union of Pure and Applied Chemistry
Calculate the pOH and the pH of the following aqueous solutions at 25°C: (a) 1.25 M LiOH (b) 0.22 M Ba(OH)2 Antonio (c) 0.085 M NaOH.
Answers
For the given aqueous solutions, the pOH and pH can be calculated as follows:a) 1.25 M LiOHpOH = 0.17, pH = 13.83. Lithium hydroxide is a strong base, thus LiOH completely dissociates in water to produce Li+ and OH- ions.
LiOH (aq) → Li+ (aq) + OH- (aq)Now, we need to find the concentration of OH- ions to determine the pOH and pH of the solution [OH-] = 1.25 M (concentration of LiOH)[OH-] = [LiOH] Thus, pOH = -log[OH-]= -log [1.25] = 0.17pH + pOH = 14, therefore pH = 14 - pOH= 14 - 0.17= 13.83b) 0.22 M Ba(OH)2pOH = 0.52, pH = 13.48 Barium hydroxide is a strong base and it completely dissociates in water into Ba2+ and 2OH- ions. Ba(OH)2 (aq) → Ba2+ (aq) + 2OH- (aq)Now, we need to determine the concentration of OH- ions:[OH-] = 2 × 0.22= 0.44 M.
Thus, pOH = -log [OH-]= -log [0.44]= 0.52pH + pOH = 14, therefore pH = 14 - pOH= 14 - 0.52= 13.48c) 0.085 M NaOHpOH = 0.93, pH = 13.07, Sodium hydroxide is also a strong base and it dissociates completely in water into Na+ and OH- ions. NaOH (aq) → Na+ (aq) + OH- (aq)The concentration of OH- ions can be found as follows:[OH-] = 0.085 MThus, pOH = -log [OH-]= -log [0.085]= 0.93pH + pOH = 14, therefore pH = 14 - pOH= 14 - 0.93= 13.07Therefore, the pOH and pH of the given aqueous solutions at 25°C are calculated as follows a) 1.25 M Li OH pOH = 0.17, pH = 13.83b) 0.22 M Ba(OH)2pOH = 0.52, pH = 13.48c) 0.085 M NaOHpOH = 0.93, pH = 13.07.
To know more about hydroxide visit:
https://brainly.com/question/31820869
#SPJ11
The pOH and the pH values of the given aqueous solutions at 25°C were calculated as given here: (a) LiOH pH = 13.83, pOH = 0.17 (b) Ba(OH)₂ pH = 13.48, OH = 0.52 (c) NaOH pH= 13.07, pOH= 0.93.
Given the concentration of LiOH = 1.25 M
The concentration of Ba(OH)₂ = 0.22 M
The concentration of NaOH = 0.085 M
In the aqueous solution, LiOH is dissociated in Li⁺ and OH⁻. The concentration of [OH⁻]= [ LiOH] ions in the solution is 1.25 M To calculate the pOH:
pOH = - log [OH⁻] = -log 1.25
=0.17
pH+pOH = 14, So the pH of LiOH is = 14 -0.17 = 13.83.
Ba(OH)₂ in aqueous solution gives Ba⁺ + 2OH⁻
Concentration of [OH⁻] = Concentration of Ba(OH)₂= 0.22 M
since on dissociation of one molecule, two OH_ ions are formed so
the concentration of OH⁻ = 2× 0.22 = 0.44
We know that pOH = -log [OH⁻] = -log 0.44 = 0.52
So the pH of an aqueous solution of Ba(OH)₂ = 14- 0.52 = 13.48
in the aqueous solution, NaOH dissociates into Na⁺ and OH⁻ ions.
[OH⁻] = [NaOH]⁻ =0.085
pOH = -log [OH⁻] = -log 0.085 = 0.93
So the pH of the NaOH solution is calculated as
14 -0.93 = 13.07
To learn more about pH, refer to the link:
https://brainly.com/question/2288405
#SPJ4
Which type of beak would be most useful to a bird that spears fish for food
long and pointed
long and curved
short and wide
short and flat short and narrow
Answers
curved beaks help so fish don’t escape
The form in which the chemical energy is stored in cells during photosynthesis
A) Photosynthesis
B)Products
C)Chloroplasts
D)Glucose
Answers
can u pls answer 22, 23, 24
Answers
Answer:
I'm terribly sorry but the download is not working for me! All it says is Download pdf, and when I click it, It does nothing except for refreshing the page!
Explanation:
CAN SOMEONE PLEASE HELP ME ??!!!!!!
Our garbage disposal techniques have changed over time. Describe two ways that a “garbage dump” from the past would differ from a more modern sanitary landfill site
Answers
Answer:
The Resource Conservation and Recovery Act (RCRA; PL 94-580), the major federal law on waste disposal, was passed in 1976. Its primary goal was to "protect human health and the environment from the potential hazards of waste disposal." RCRA is also concerned with reducing the amount of waste generated, ensuring that wastes are managed properly, and conserving natural resources and energy. The RCRA regulates solid waste, hazardous waste, and underground storage tanks
Explanation:
Why is Gibbs free energy negative?
Answers
A negative Gibbs free energy indicates that there is more free energy in the reactants, or beginning state, than in the products, or end state. Exergonic reactions, which may take place without the supply of energy, are also known as spontaneous reactions.
The reaction is spontaneous at high temperature if both H and S are positive, and spontaneous at low temperature if both H and S are negative. G will always be negative if H is negative and S is positive. In the case of a spontaneous reaction, Gibbs free energy is negative (only). For reactions that are not spontaneous, it can also be advantageous. We can easily anticipate the spontaneity of a reaction using the enthalpy and entropy numbers thanks to the Gibbs equation. In an exothermic process,
To learn more about Gibbs free energy please click on below link
https://brainly.com/question/20358734
#SPJ4
the name of Cu(C2H3O2)2
Answers
Answer:
The name of the compound Cu(C2H3O2)2 is Copper(II) acetate.
In this name "Copper" refers to the element copper (Cu) and the "II" in parentheses refers to the oxidation state of copper which is +2. "Acetate" is the anion name of C2H3O2, which is the anion present in the compound.
The IUPAC name of the compound Cu(C\(_2\)H\(_3\)O\(_2\))\(_2\) is Copper(II) acetate. In this name, "Copper" means the chemical element copper (Cu), and the "II" in brackets stands for copper's +2 oxidation state.
Whether either an ongoing link or a ring, the greatest number of carbons joined by a single bond serves as the basis for IUPAC nomenclature. According to a certain set of priorities, any deviations, whether multiple bonds and atoms other than hydrogen and carbon, are denoted by prefixes or suffixes. In this name, "Copper" means the chemical element copper (Cu), and the "II" in brackets stands for copper's +2 oxidation state. The compound's anion, C\(_2\)H\(_3\)O\(_2\), is known by its anion name "acetate".
To know more about IUPAC, here:
https://brainly.com/question/16631447
#SPJ6
The first ionization energies of the elements ______ as you go from left to right across a period of the periodic table, and ______ as you go from the bottom to the top of a group in the table.
A.) increase, decrease
B.) decrease, increase
C.) decrease, decrease
D.) unpredictable, unpredictable
E.) increase, increase
Answers
The correct answer to the question is: A) increase, decrease
The first ionization energies of the elements increase as you go from left to right across a period of the periodic table, and decrease as you go from the bottom to the top of a group in the table.
1. Going from left to right across a period, the atomic number increases, which means there are more protons in the nucleus. This results in a stronger attraction between the positively charged nucleus and the negatively charged electrons. As a result, it becomes harder to remove an electron, requiring more energy, and therefore the first ionization energy increases.
2. Going from the bottom to the top of a group, the atomic size decreases. This is because the number of energy levels or shells decreases, and the electrons are closer to the nucleus. As the distance between the nucleus and the outermost electrons decreases, the attractive force between them increases. Consequently, it becomes easier to remove an electron, requiring less energy, and therefore the first ionization energy decreases.
Therefore, the correct answer to the question is:
A) increase, decrease
To know more about protons, visit:
https://brainly.com/question/12535409
#SPJ11
Lithium seems to have dramatic effects on the nervous system, yet our bodies seemingly never evolved to use lithium endogenously, rather focusing on sodium, potassium, and calcium. what is a plausible explanation for this?
Answers
Given its small size and high polarizing power, lithium is only involved in covalent rather than ionic bonds in the body as does sodium, potassium, and calcium.
What is lithium?Lithium is a metal and a member of group 1 in the periodic table. It is a very small ion hence it has a different chemistry from the rest of the members of the group.
Lithium is not part of any of the biomolecules because given its small size and high polarizing power, it is only involved in covalent rather than ionic bonds in the body as does sodium, potassium, and calcium.
Learn more about lithium:https://brainly.com/question/4219437?r
How could you test whether or not a substance is a fluid?
Answers
Why is water so important for life?Why cant other substance made of hydrogen and oxygen like hydrogen peroxide take its place?
Answers
Answer:
Reasons water is important
Imagine earth without water. The soil, with no water in it and nothing growing on it, would be lifeless, dead, collapsed into dust, sand, clay or rock.
-Water consumption helps lubricate and cushion your joints, spinal cord, and tissues. This will help you enjoy physical activity and lessen discomfort caused by conditions like arthritis.
-Water is a main component of saliva. Saliva also includes small amounts of electrolytes, mucus, and enzymes. It’s essential for breaking down solid food and keeping your mouth healthy.
When one substance attracts and combines with another substance to form a uniform solution, the process is called ___
a. Absorption b. Adsorption
c. Subcooling d. Subheating
Answers
When one substance attracts and combines with another substance to form a uniform solution, the process is called Adsorption. The correct answer is b.
Adsorption refers to the process in which one substance is attracted to and combines with another substance to form a uniform solution. In adsorption, the molecules of the adsorbate (the substance being attracted) adhere to the surface of the adsorbent (the substance attracting). This process occurs through weak intermolecular forces such as van der Waals forces or hydrogen bonding. Adsorption is commonly observed in various fields, such as chemistry, biology, and environmental science, and plays a significant role in processes like chromatography, catalysis, and water treatment. Hence the correct answer is b.
To know more about Adsorption, here
brainly.com/question/31087308
#SPJ4
Choose all that are correct about the elements in the column highlighted in blue
in the periodic table below (there are FOUR correct answers)
help !!!
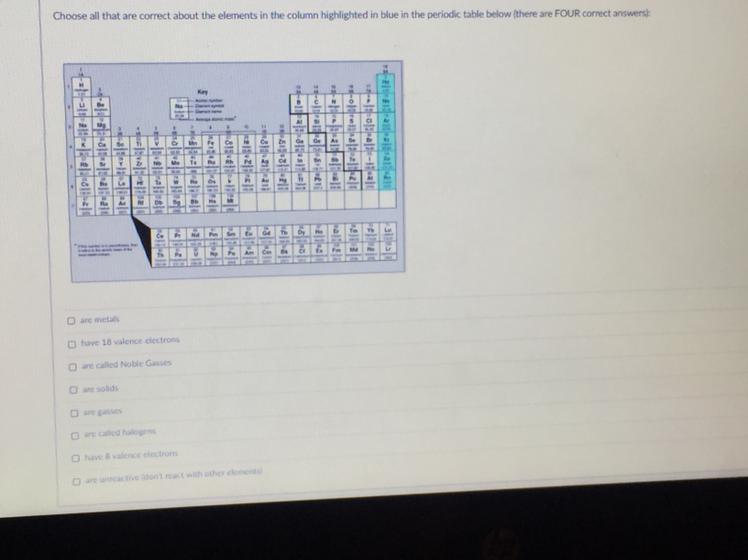
Answers
Answer:
running a business is the best i have ever done to make a good friend of yours to get to the point where you have a lot to say
Which of the following is true of the relationships between heat of fusion and heat of vaporization?
A.) Using the heat of fusion, you can predict the heat of vaporization.
B.) The heat of vaporization is typically larger than the heat of fusion.
Answers
Answer:
The heat of vaporization is typically larger than the heat of fusion
Next question answer:
The liquid water absorbs heat from the skin surface and is transferred to the air when the water evaporates.
Explanation:
Answer:
First answer
B. The heat of vaporization is typically larger than the heat of fusion
Second answer:
A. The liquid water absorbs heat from the skin surface and is transferred to the air when the water evaporates.
Explanation:
Which best explains why conservation of mass should be studied in a closed
system?
A. to prevent contamination of the products
o
B. to prevent the loss of any reactants
c. to prevent the release of any gases produced
D. to prevent the absorption of heat from the outside
Answers
Answer: C
Explanation:
The option that best explains why conservation of mass should be studied in a closed system is to prevent the release of any gases produced.
The law of conservation of mass states that mass can neither be created nor destroyed in a reaction, but can be converted from one form to another during the course of the reaction.
A closed system is one that cannot exchange matter with its surrounding while an open system is free to exchange both matter and energy with its surrounding.
When a system is opened, gaseous reactants of products may be lost to the surroundings and the mass will not be conserved as a result.
However, in a closed system, gaseous reactants or products remain within the system and the mass of the system before and after the reaction remains constant.
More on the law of conservation of mass can be found here: https://brainly.com/question/13383562
How many moles of Neon atoms are in 224 liters of Neon gas under STP?
Answers
The sketch below shows two marbles . The arrows show the size and the direction of the momentum of the two marbles . Draw arrows in the space below that show what will happen to these two marbles because of the law of conservation of momentum when they collide

Answers
The arrows representing the momentum of the marbles will reflect the conservation of momentum principle, where the total momentum of the system is conserved before and after the collision.
What is the final momentum of the marbles after the collision?Let the bigger marble = ALet the smaller marble = BBased on the information provided, the bigger marble (A) is moving to the right and has momentum in that direction. The smaller marble (B) is moving to the left and has momentum in that direction. When they collide, according to the law of conservation of momentum, the total momentum of the system will remain constant.
Therefore, after the collision:
Marble A (bigger) will continue to move to the right, but with a reduced momentum, as some of its momentum will be transferred to Marble B during the collision. The arrow representing the momentum of Marble A will be smaller in size than the initial arrow, but still pointing to the right.
Marble B (smaller) will change its direction and start moving to the right, as some of the momentum from Marble A will be transferred to it during the collision. The arrow representing the momentum of Marble B will be larger in size than the initial arrow, and pointing to the right.
Learn more about conservation of momentum here: https://brainly.com/question/7538238
#SPJ1
Consider the combustion of propane:
C3H8 (g) + 5O2 (g) ⟶ 3CO2 (g) + 4H2O(l) ΔH = –2221 kJ
Assume that all the heat comes from the combustion of propane. What mass of propane must be burned to furnish this amount of energy assuming the heat transfer process is 60.% efficient?
Answers
73.66 grams of propane must be burned to furnish the required amount of energy, assuming a 60% heat transfer efficiency.
To determine the mass of propane needed to furnish the required amount of energy, follow these steps:
1. Identify the given information:
ΔH (heat of combustion) = -2221 kJ/mol
Efficiency = 60%
2. Calculate the actual energy required:
Since the heat transfer process is 60% efficient, we need to account for that when determining the energy needed.
Energy required = -2221 kJ / 0.60 = -3701.67 kJ/mol
3. Determine the mass of propane:
Now, we'll use the energy required and the given balanced chemical equation to find the mass of propane.
First, find the molar mass of propane (C3H8). C = 12.01 g/mol, H = 1.01 g/mol.
Molar mass of C3H8 = (3 × 12.01) + (8 × 1.01) = 36.03 + 8.08 = 44.11 g/mol
Next, divide the energy required by the heat of combustion:
Moles of propane = -3701.67 kJ / -2221 kJ/mol = 1.67 mol
Finally, multiply the moles of propane by the molar mass to find the mass of propane needed:
Mass of propane = 1.67 mol × 44.11 g/mol = 73.66 g
So, 73.66 grams of propane must be burned to furnish the required amount of energy, assuming a 60% heat transfer efficiency.
Learn more about propane here:
https://brainly.com/question/14519324
#SPJ11
when silver is mixed with aluminum nitrate the products are silver nitrate and aluminum metal. how many molocules of silver nitrate are produced if we have 216 grams of aluminum nitrate?
STOICHIOMETRY
Answers
Answer:Aluminum metal in silver nitrate ….
You might be expecting silver metal, Ag, and aluminum nitrate, Al(NO3)3, as products. But instead all you will end up with is wet aluminum. The passivating layer of Al2O3 will prevent aluminum from reducing silver ions.
Explanation: Al(s) + AgNO3(aq) → No reaction
The two things we might do to the mixture to disrupt the Al2O3 surface will cause the Ag+ ions to react. Often a source of chloride ion is used because AlCl4^- will form, exposing the aluminum surface. But that will cause AgCl(s) to precipitate. You can also make the solution basic with NaOH and form Al(OH)4^-, but that will cause Ag2O(s) to form. (You will get Ag2O instead of AgOH.)
Concentrated acids will oxidize any aluminum metal that might be exposed if you were to add concentrated HNO3. That would simply enhance the
When mixing 5.0 moles of HZ acid with water up to complete a volume of 10.0 L, it is found that at
reach equilibrium, 8.7% of the acid has become hydronium. Calculate Ka for HZ. (Note: Do not assume is disposable. )a. 1.7×10^−3
b. 9.5×10^−2
C. 2.0×10^−2
d. 4.1×10^−3
e. 3.8×10^−3
f. 5.0×10^−1
Answers
therefore the correct option is d) 4.1×10⁻³.
Given that the initial concentration of HZ is 5.0 moles and at equilibrium, 8.7% of the acid has become hydronium.
The concentration of HZ that has not reacted is (100% - 8.7%) = 91.3%.
The final concentration of HZ is 5.0 × 0.913 = 4.565 moles.
The final concentration of the hydronium ion is 5.0 × 0.087 = 0.435 M.HZ ⇌ H+ + Z-Ka
= [H+][Z]/[HZ]Ka
= [H+][Z]/[HZ]
= [0.435]² / 4.565
= 0.041
Which is the same as 4.1 × 10-3.
We know that HZ is an acid that will partially ionize in water to give H+ and Z-.
The chemical equation for this reaction can be written as HZ ⇌ H+ + Z-.
The acid dissociation constant (Ka) of HZ is the equilibrium constant for the reaction in which HZ ionizes to form H+ and Z-.Thus, Ka = [H+][Z]/[HZ].
The given problem is a typical example of the dissociation of a weak acid in water. We are given the initial concentration of HZ and the concentration of hydronium ions at equilibrium.
To find the equilibrium concentration of HZ, we can use the fact that the total amount of acid is conserved.
At equilibrium, 8.7% of HZ has dissociated to give hydronium ions.
This means that 91.3% of the original HZ remains unreacted.
Therefore, the concentration of HZ at equilibrium is 5.0 × 0.913 = 4.565 M.
The concentration of hydronium ions at equilibrium is 5.0 × 0.087 = 0.435 M.
Using the equation Ka = [H+][Z]/[HZ], we can substitute the values of the concentrations and the equilibrium constant into the equation and solve for Ka.
Ka = [H+][Z]/[HZ]
= [0.435]² / 4.565
= 0.041 or 4.1 × 10-3.
The answer is d) 4.1 × 10-3.
To know more about hydronium visit:
https://brainly.com/question/14619642
#SPJ11
An acid is:
a proton acceptor
any substance that reacts to keep blue litmus blue
any compound containing hydrogen
a proton donor
a compound having hydrogen as a cation and oxygen as an anion.