calculate the number of grams of aluminum required to prepare 299.0 g of chromium metal by the first reaction.
Answers
The mass of aluminum required to prepare 297.0g of chromium is 154.25 g.
The formation of chromium using aluminum powder is an exothermic process as heat is evolved in this process. In this type of reaction heat is released during the formation of product.
The balanced chemical reaction for the formation of chromium is given as,
Cr₂O₃(s) + 2Al(s) -> 2CrO(s) + Al₂O₃(s)
Thus, we can see that 2 moles of aluminum produces 2 moles of chromium.
Hence,
Molar mass of aluminum = 27g
∴ 2 moles of aluminum is required then mass of aluminum is 2 x 27 = 54g.
Molar mass of chromium = 54g
∴ 2 moles of chromium is obtained then mass of chromium is 2 x 54 = 104g.
Therefore, the amount of aluminum required,
mass of aluminum produced = 54 x 299/ 104
= 16146/104 = 155.25 g.
Therefore, the required amount of aluminum is 154.25 g.
Learn more about the formation of chromium from the link given below.
https://brainly.com/question/29761513
#SPJ4
Related Questions
6.Find the empirical formula of a compound that contains:19.16 g Sodium1.680 g Hydrogen25.81 g Phosphorus
Answers
The empitical formula shows the simplest ratio of elements in a compound (not the total number of atoms in the molecule).
So to find the empirical formula we need to calculate how many moles of each atom we have in this sample. Then we will see the ratio of each element.
We are given the mass, so to convert it to moles we use the molar mass. For this we go to the periodic table and see that the values for each element are:
Na (sodium): 22,99 g/mol
H (hydrogen): 1 g/mol
P (phosphorus): 25,81 g/mol
So we calculate the moles of each element as follows:
\(\begin{gathered} moles_{Na}=\frac{Mass_{Na}}{Molar\text{ }mass_{Na}}=\frac{19.16g}{22.99g/mol}=0.833\text{ mol} \\ moles_H=\frac{Mass_H}{Molar\text{ }mass_H}\text{ }=\frac{1.68g}{1g/mol}=1.68mol \\ moles_P=\frac{Mass_P}{Molar\text{m}ass_P}\text{=}\frac{25.81g}{30.97\frac{g}{mol}}=0.833mol \end{gathered}\)And as we see, for every 0.833 moles of Na we have the same number of moles of P, so the ratio of these elements in the molecule is 1 to 1.
As for the hydrogen:
\(\frac{moles_{Na}}{moles_H}=\frac{0.833}{1.68}\approx\frac{1}{2}\)So the ratio Na to H is 1 to 2.
Now we can write the empirical formula as follows=
\(NaH_2P\)
What was the purpose of rinsing with hexanes in the Cyalume synthesis procedure? What was the purpose of rinsing with water in the Cyalume synthesis procedure? Why is it important/useful to prepare active ingredients that contain an amino group as ammonium chloride salt? Draw (chemdraw) the whole mechanism of bupropion HCl synthesis
Answers
In this course of union of Cyalume, the motivation behind flushing with hexane" is the filtration of Response mass for precipitate take the strong and give washing with more.
Hexane and their reaction to the filter Mass and to acquire more pure products. This product was not dissolved in the solvent hexane, resulting in a brown color and product.
b) In this Synthesis of Cyalume 1 process, the purpose of rinsing with water is to wash the product with water and filter it before washing it with hexane to make a slurry and get a brown color precipitate.
Ammonium chloride is a white glasslike strong. Water dissolves it (37%). The environmental threat is the primary danger. It is necessary to take immediate measures to stop its spread to the environment. It is utilized to make other ammonium compounds, as a welding motion, as a manure, and for the vast majority different purposes.Ammonium chloride salt :Ammonium chloride is a salt that makes the body and the urine more acidic. Ammonium chloride keeps up with pH and applies a gentle diuretic impact. This acid-forming salt is used to treat coughs because it also has an expectorant effect by irritating the mucous membranes.
Ammonium is the counterion in ammonium chloride, an inorganic chloride. It plays a role as an inhibitor of ferroptosis. It is an inorganic chloride and an ammonium salt.
Ammonium chloride can be found in cleanser, hair tone and fade, body wash and cleaning agent, facial chemical, conditioner, hand dishwashing cleanser, as well as in shower oils and salts. Because it is an ionic compound, ammonium chloride is utilized as an important electrolyte in dry cell batteries.
Learn more about ammonium chloride salt :
brainly.com/question/12969993
#SPJ4
In the Cyalume synthesis procedure, rinsing with hexanes is performed to remove impurities and unreacted starting materials that are soluble in hexanes.
Hexanes is a nonpolar solvent that can effectively dissolve nonpolar compounds while leaving behind the desired product or polar impurities. By rinsing with hexanes, the impurities can be separated from the desired product, improving the purity of the final product.
Rinsing with water in the Cyalume synthesis procedure is carried out to eliminate water-soluble impurities. Water can dissolve polar compounds, including water-soluble impurities, while the desired product may remain insoluble or less soluble. Rinsing with water aids in the purification process by removing these impurities and enhancing the quality of the final product.
Preparing active ingredients containing an amino group as ammonium chloride salt is important and useful for several reasons. Ammonium salts increase the stability of such compounds, prolonging their shelf life. They also tend to have improved solubility in water compared to their free base counterparts, facilitating formulation and enhancing bioavailability. The ionic interactions of ammonium salts with other charged species can affect the pharmacokinetics and pharmacodynamics of the active ingredient.
Furthermore, the solid-state properties of ammonium salts make them favorable for manufacturing dosage forms like tablets or capsules, due to improved crystallinity, flowability, and compressibility. These factors collectively contribute to the efficacy, stability, and ease of formulation of active ingredients containing an amino group as ammonium chloride salt.
To know more about the Cyalume synthesis refer here :
https://brainly.com/question/32092304#
#SPJ11
Question 4
The analysis of gas and how it behaves has been undertaken to develop several gas laws. Using applicable gas laws establish solutions for the following
a) a mass of gas has a pressure of 450 kPa and temperature of 140°C. The pressure is doubled during a process but the volume remains unchanged. What is the new temperature so cooling systems can be designed?
b) a mass of gas at a temperature of 160°C has a volume of 0.2m³ is cooled down by 110°C with no change in pressure. Calculate the new volume of the gas.
Answers
A mass of gas has a pressure of 450 kPa and temperature of 140°C. The pressure is doubled during a process but the volume remains unchanged.
In order to solve this problem, we need to apply Charles' Law: V1/T1 = V2/T2. Since the volume remains unchanged, we can simplify this to T1/P1 = T2/P2. T1 and P1 are the initial temperature and pressure, respectively. T2 is the unknown final temperature, and P2 is double the initial pressure (i.e., 2P1).
Substituting the given values:140 + 273 = 413 K450 kPa * (2) = 900 kPa413 K/450 kPa = T2/900 kPaT2 = (413 K / 450 kPa) * (900 kPa) = 756 KWe must then subtract 273 to convert from kelvin to Celsius. Therefore, T2 = 483°C, which is the new temperature.
In this case, the gas law to apply is Charles’ law which states that at a constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature. The general equation of Charles' law is V1/T1 = V2/T2, where V is the volume of the gas, T is the temperature, and the subscripts 1 and 2 denote initial and final states, respectively. For our question, since the volume remains unchanged, we can simplify this to T1/P1 = T2/P2. T1 and P1 are the initial temperature and pressure, respectively. T2 is the unknown final temperature, and P2 is double the initial pressure (i.e., 2P1).
Therefore, T2 = (T1 x P2)/P1. We can substitute the given values into the formula and solve for T2 as follows.
140 + 273 = 413 K450 kPa x 2 = 900 kPa
T2 = (413 K x 900 kPa)/450 kPa = 826 K
Subtracting the value of absolute zero (273) from 826, we obtain T2 = 553°C. This is the final temperature of the gas after doubling the pressure.
Learn more about absolute temperature: https://brainly.com/question/16269132
#SPJ11
The smallest atomic unit which maintains the physical properties of a compound is a(n)
Answers
Answer:
The smallest atomic unit which maintains the physical properties of a compound is called a molecule.
Explanation:
A molecule is the smallest unit of a compound that can exist independently and still retain the properties of that compound. It is made up of two or more atoms that are bonded together, and it is the basic unit of a chemical compound. The atoms in a molecule are held together by chemical bonds, which are the attractive forces that hold the atoms together. Molecules can be made up of different types of atoms, and the type and arrangement of atoms in a molecule determine the properties of the compound.
Explain why an organism dies if the respiratory and circulatory system 'paused' for a while.
Answers
Answer:
Without the respiratory system your blood would be useless. The circulatory and respiratory systems work together to circulate blood and oxygen throughout the body
Explanation:
Which bases can be used to deprotonate a terminal alkyne? Choose all that apply. A. LICH3 B. NaNH2 NaH D. KOC(CH3)3
Answers
To deprotonate a terminal alkyne, we need a strong base that can remove the acidic hydrogen from the terminal carbon. The bases that can be used for this purpose are LICH3, NaNH2, NaH, and KOC(CH3)3. All of these bases are strong enough to remove the acidic hydrogen from the terminal carbon of an alkyne.
However, the choice of base depends on the specific reaction conditions and the desired outcome. For example, LICH3 is a highly reactive base and is often used in reactions that require a fast and strong deprotonation step. On the other hand, NaH is a milder base that is often used in reactions that require a slower and more controlled deprotonation step. Therefore, it is important to consider the specific reaction conditions and the desired outcome when choosing a base to deprotonate a terminal alkyne. we can conclude that different bases have different strengths and properties, which make them suitable for different types of reactions. It is important to understand the properties of each base and the conditions under which they are most effective to choose the right base for a specific reaction.
To know more about alkyne visit:
https://brainly.com/question/30901211
#SPJ11
can somebody please help me with this question please
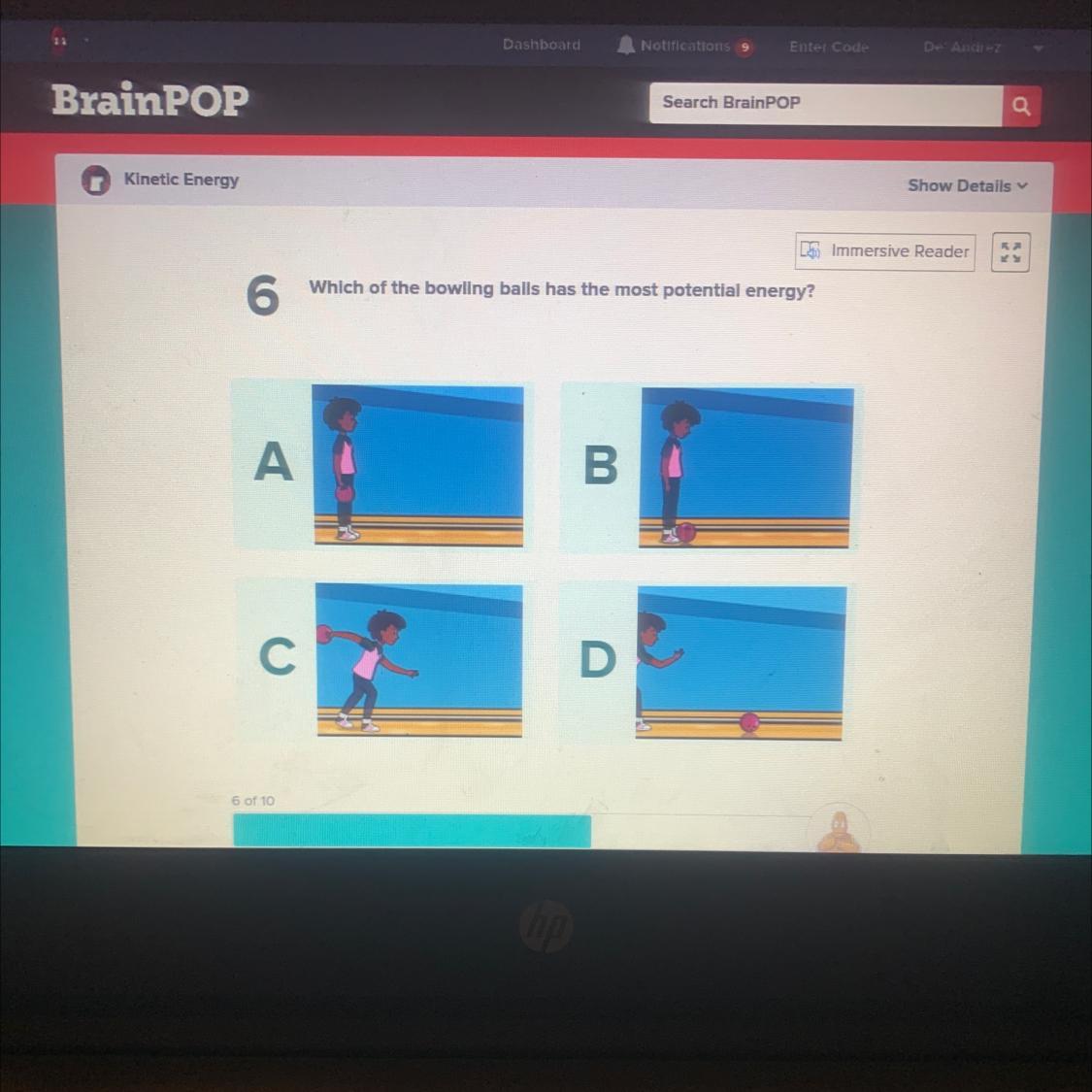
Answers
Answer:
c because she crating the energy
Help me please I need this quickly

Answers
9. melting point
10. balloons float because they are filled with hydrogen, a gas which is less dense than air.
11. specific gravity
12. element
13. mixture
14. sodium and chlorine
15. cutting of paper
16. condensation
17. 1 and 3
18. heat energy is released
A student has one mole of zinc and one mole of oxygen. Which of the following is true for both samples? *
There are equal masses of zinc and oxygen
There are equal numbers of atoms of zinc and oxygen
There are equal volumes of zinc and oxygen
There are equal densities of zinc and oxygen
Answers
Answer:
2
Explanation:
what determines the role of an organism in an ecosystem?

Answers
Answer:
An organism's role within an ecosystem depends on how it obtains its food. Plants and animals obtain their food in very different ways, so they have very different roles in an ecosystem. The way in which an organism obtains food also affects its interactions with other organisms in the ecosystem.
Explanation:
Simplify - Factor a
Which particles represent a solid? *
Option 1
Option 2
Option 3
Option 4 - Surface of sun

Answers
Answer:
Option 1
Explanation:
Option number 1 is the most compact one which indicates it is a solid
You have 150 g sugar solution the percent by mass of the solute sugar is 15% how many grams of sugar are there in the solution
Answers
Answer:
B George Washington
Explanation:
Hope it helps
Uranium has three common isotopes. If the abundance of 234u is 0. 01%, the abundance of 235u is 0. 71%, and the abundance of 238u is 99. 28%, what is the average atomic mass of uranium?.
Answers
The average atomic mass of uranium is 237.97 amu based on its three common isotopes.
Isotopes are the different forms of the same element. In considering the abundance of different isotopes of uranium for the calculation of its average atomic mass, the formula below is used.
Average atomic mass = summation of [(percent abundance /100)*atomic mass]
With the given three common isotopes of uranium, the calculated value of average atomic mass is shown below.
Average atomic mass= (0.01/100)(234 amu) + (0.71/100)(235 amu) + (99.28/100)(238 amu)
Average atomic mass=237.97 amu
For more information regarding isotopes, please refer to the link https://brainly.com/question/6258301.
#SPJ4
Write the nuclear symbol for 25p, 30n, 21e
Answers
Answer:
Explanation:The atom with atomic number 17 and mass number 35 is chlorine. Its complete symbol is
17
35
Cl.
(ii) The atom with atomic number 92 and mass number 233 is uranium. Its complete symbol is
92
233
U.
(iii) The atom with atomic number 4 and mass number 9 is beryllium. Its complete symbol is
4
9
Be.
What transformations take the graph of f(x) = eº to f(x) = −eª – 2?
Answers
Vertical Shift: Down 2 Units
Reflection about the x-axis: Reflected about x = a.
What is transformation on the graphs?Let the functions f(x) and g(x) be two real functions.
And g (x) = f(x) + k, where k is real numbers.
The function can be sketched by shifting f (x), k units vertically.
The direction of shift can be found by the value of k:
if k > 0, the base graph shifts k units up, and
if k < 0, the base graph shifts k units down.
Given:
A parent function,
f(x) = eº.
And transformed graph g(x) = −eª – 2.
Parent Function: eˣ
Horizontal Shift: None
Vertical Shift: Down 2 Units
Reflection about the x-axis: Reflected about x = a.
Vertical Compression or Stretch: None
Therefore, vertical shift: Down 2 Units
To learn more about the transformation on the graphs;
https://brainly.com/question/19040905
#SPJ9
environmental agents such as nutrition and chemical exposure can alter gene expression by affecting the epigenome. epigenetic changes may also have important effects on behavior phenotypes. which of these examples correctly describe explicit evidence supporting epigenetic traits as heritable?
Answers
An example correctly describing explicit evidence supporting epigenetic traits as heritable is DNA methylation.
The epigenome can be impacted by environmental factors like a person's nutrition and exposure to contaminants. When cells divide, epigenetic alterations can be preserved from cell to cell and, in some situations, passed down through the generations. DNA methylation is a frequent epigenetic alteration type.
DNA methylation is an epigenetic modification, meaning it affects DNA without changing its sequence in any way. It modifies a gene's expression during cell differentiation and brings about a heritable alteration. Other potential environmental stressors that can alter epigenetic status include chemical and xenobiotic compounds in water or the atmosphere.
To know more about epigenome, refer to the following link:
https://brainly.com/question/16319638
#SPJ4
what element is located in group 5, period 4?
Answers
Answer:
vanadium
Explanation:

How many moles of solute would you need to add to 500mL solution to get a
molarity of 3.7 mol/L?
Answers
Answer:
7.4 moles solute
Explanation:
From definition of Molarity (M) = moles solute (n)/volume of solution in Liters(V)
=> moles solute (n) = Molarity (M) x Volume (L) = (3.7 moles/L)(0.500L) = 7.4 moles solute
9) Is there any other ratio of aluminum and oxygen ions that could exist?
For instance, could you have Alz0 or AlO2? Explain your answer.
Answers
Answer:
The ratio of aluminium and oxygen ions that only exists is 2:3
Since Aluminium has 3 valence electrons and oxygen has 2 vacant orbitals
Aluminium holds a valence of 3 and oxygen 2, when they react a compound of formula
\(Al _{2} O _{3}\)
is formed
According to the electronic configuration and valency, ratio that could exist between aluminium and oxygen is 2:3.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/13497372
#SPJ2
what color and texture are best r reflectors of radian heat
Answers
Answer:
White colors and white surfaces because white surfaces are poor absorbers and good reflectors of heat radiation.
Explanation:
Hope it helps! ❤️
the answers that can be written down are at the bottom

Answers
Answer:
increasing / protons / periods / groups / center / far right / similar
An element that is malleable and a good conductor of heat and electricity could have an atomic number of
Answers
Answer:
Copper (atomic number 29)
Explanation:
Copper is a element that is malleable and a good conductor of heat and electricity.
a reaction has a δhrxn = 23.25 kj and δs was 161.26 j/mol∙k. this reaction is spontaneous
Answers
The spontaneity of a reaction is determined by the Gibbs free energy change (∆G), which is a measure of the system's ability to do work.
The equation that links ∆G, ∆H, and ∆S is: ∆G = ∆H - T∆S, where T is the temperature in Kelvin and ∆H and ∆S are the enthalpy and entropy changes, respectively. The signs of ∆H and ∆S determine whether the reaction is exothermic or endothermic and whether it is entropy-driven or enthalpy-driven, respectively.
If ∆G is negative, the reaction is spontaneous, meaning it occurs without the input of energy.The given reaction has a ∆H of 23.25 kJ and a ∆S of 161.26 J/mol∙K.
First, we need to convert the units of ∆S from J/mol∙K to kJ/mol∙K by dividing by 1000.∆S = 161.26 J/mol∙K ÷ 1000 = 0.16126 kJ/mol∙K Substitute the values into the equation to determine the spontaneity of the reaction:
∆G = ∆H - T∆S∆G = (23.25 kJ) - (298 K) x (0.16126 kJ/mol∙K)∆G = 23.25 kJ - 48.02 kJ∆G = -24.77 kJ Since ∆G is negative, the reaction is spontaneous.
To know more about Gibbs free energy refer here: https://brainly.com/question/13795204#
#SPJ11
according to the reference tables for physical setting/chemistry, which metal would react spontaneously with hydrochloric acid?
Answers
According to the reference tables for Physical Setting/Chemistry, the metal that would react spontaneously with hydrochloric acid (HCl) is magnesium (Mg).
How does magnesium react with hydrochloric acid?Magnesium and hydrochloric acid combine to create hydrogen gas and magnesium chloride. As a result, the metal is oxidized to a +2 oxidation state, releasing hydrogen gas from the acid as it dissolves. In contrast, metals with higher electro positivity than hydrogen will react spontaneously with hydrochloric acid, such as calcium, strontium, and barium.
In the reactivity series of metals, magnesium is more reactive than hydrogen. When a metal is more reactive than hydrogen, it will displace hydrogen from an acid such as hydrochloric acid (HCl) and undergo a spontaneous reaction. This type of reaction is known as a displacement reaction.
When magnesium reacts with hydrochloric acid, the following reaction occurs:
Mg + 2HCl → MgCl₂ + H₂
In this reaction, magnesium displaces hydrogen from hydrochloric acid, resulting in the formation of magnesium chloride (MgCl₂) and the release of hydrogen gas (H₂). The reaction is spontaneous because magnesium is more reactive than hydrogen.
It's important to note that the reactivity series of metals ranks metals in order of their reactivity, with the most reactive metal at the top. Metals higher in the reactivity series can displace metals lower in the series from their compounds during chemical reactions. Hence, magnesium, being higher in the reactivity series than hydrogen, can displace hydrogen from hydrochloric acid.
In conclusion, magnesium (Mg) is the metal that react spontaneously with hydrochloric acid (HCl).
Please refer to the specific reference tables for Physical Setting/Chemistry for further details and comprehensive information on the reactivity series of metals and their reactions with acids.
Learn more about magnesium here:
https://brainly.com/question/5759562
#SPJ11
Which is a form of kinetic energy?
gravitational energy
chemical energy
electrical energy
sound energy
DO
Answers
Answer:
Electrical
There are five types of kinetic energy: radiant, thermal, sound, electrical and mechanical.
Which is one factor that adds to the greenhouse effect?
a decrease in the amount of dust in the atmosphere
a decrease in the amount of water on Earth’s surface
an increase in gases in the atmosphere that absorb heat
an increase in the amount of solar radiation that reflects into space
Answers
Answer:
C
Explanation:
Edge 2022 trust the process
Knowing about the greenhouse effect we have that what intensifies this effect is an increase in gases in the atmosphere that absorb heat.
What is the greenhouse effect?Greenhouse effect is a natural atmospheric phenomenon responsible for the maintenance of life on Earth. Without the presence of this phenomenon, the temperature on Earth would be very low, around -18ºC, which would make the development of living beings impossible.
Thus, the main cause of the greenhouse effect is industrialization. Due to this process, the burning of fossil fuels has intensified in recent centuries. The main products of the combustion reaction of these reagents are the gases mentioned above as the main ones for the greenhouse effect.
See more about greenhouse effect at brainly.com/question/1577730
Amino acids that are usually positive, i.e. Protonated, at physiological pH
Answers
There are several amino acids that are usually positive, or protonated, at physiological pH, which is around 7.4. These include histidine, lysine, and arginine.
Histidine has a side chain with a pKa of approximately 6.0, which means that at physiological pH, about half of the histidine molecules will be protonated and carry a positive charge. Lysine and arginine have side chains with even higher pKa values, around 10.8 and 12.5, respectively. As a result, almost all of the lysine and arginine molecules in a physiological environment will be protonated and positively charged. These positively charged amino acids play important roles in protein structure and function, as well as in enzyme catalysis and ion transport across cell membranes.
Amino acids that are usually positive or protonated at physiological pH (around 7.4) are lysine, arginine, and histidine. These amino acids contain basic side chains which can accept protons, making them positively charged under physiological conditions.
Visit here to learn more about amino acids brainly.com/question/28409615
#SPJ11
Where in Recycle City where you can get information on what to do with leftover cleaning products. Why is it important that we not throw chemicals into the regular trash
Answers
In Recycle City, you can get information on what to do with leftover cleaning products at the Household Hazardous Waste Facility.
It is important that we not throw chemicals into the regular trash because they can be harmful to the environment and human health. Chemicals can leach into the soil and groundwater, contaminating water sources and harming wildlife. They can also release toxic gases when burned in incinerators or landfills.
By properly disposing of leftover cleaning products, we can prevent these harmful effects and protect the environment. The Household Hazardous Waste Facility is designed to handle these types of materials and can safely dispose of or recycle them. It is important to follow proper disposal guidelines to ensure the safety of ourselves and our community.
To know more about the Trash, here
https://brainly.com/question/30362573
#SPJ1
PLEASE ANSWER QUICKLYYY
At standard temperature and pressure one mole of H2 gas occupies
22.4 L. What is the volume occupied by 3.50 moles of H2?
Answers
Answer 78.4
Explanation:
Idfk calculations
context givin in picture

Answers
Answer:
200
Explanation: