Answers
Answer:
0.48g
Explanation:
Given parameters:
Mass of zinc = 15g
Unknown:
Mass of hydrogen gas = ?
Solution:
To solve this problem, we need to state the balanced reaction expression first;
Zn + 2HCl → ZnCl₂ + H₂
Find the number of moles of Zn;
Number of moles = \(\frac{mass}{molar mass}\) = \(\frac{15}{63.4}\) = 0.24mol
From the balanced reaction expression;
1 mole of Zn will produce 1 mole of H₂
0.24mole of Zn will produce 0.24mole of hydrogen gas
Mass of hydrogen gas = number of moles x molar mass
= 0.24 x (2 x 1)
= 0.48g
Related Questions
V1 = 5 L P1 = 0.5 atm V2 = 2.5 L P2 = ..........

Answers
Answer
1 atm
Explanation
Given:
V₁ = 5 L P₁ = 0.5 atm V₂ = 2.5 L P₂ = ?
According to Boyle's law, the pressure (P) of a given quantity of gas varies inversely with its volume (V) at constant temperature; i.e., in equation form,
P₁V₁ = P₂V₂
So substitute the given values into the equation to get P₂
0.5 atm x 5 L = P₂ x 2.5 L
2.5 atm.L = P₂ 2.5 L
Divide both sides by 2.5 L
\(\begin{gathered} \frac{2.5\text{ atm}\cdot L}{2.5\text{ L}}=\frac{P_{2}2.5\text{ L}}{2.5\text{ L}} \\ P_{2}=1\text{ atm} \end{gathered}\)Which of the following is a possible
way to describe the SO3 component in
the reaction below?
Sa(s) + 120₂(g) → 8SO3(g)
A. 8 atoms SO3
B. 8 molecules SO3
C. 80.07g SO3
D. 32 LSO3
Answers
The correct answer is B. 8 molecules \(SO_3\). Option B
In the given reaction:
S(s) + \(O_2\)(g) → \(SO_3\)(g)
The stoichiometric coefficient in front of the \(SO_3\)molecule is 8, which indicates that 8 molecules of \(SO_3\)are formed as a product. This coefficient represents the ratio of the number of molecules involved in the reaction.
Option A (8 atoms \(SO_3\)) is incorrect because it only mentions the number of atoms, not molecules. The stoichiometric coefficient does not represent the number of atoms, but rather the number of molecules.
Option C (80.07g \(SO_3\)) is incorrect because it mentions a specific mass. The stoichiometric coefficient does not directly represent the mass of the substance, but rather the relative amount of molecules involved in the reaction.
Option D (32 \(SO_3\)) is incorrect because it mentions a specific volume. The stoichiometric coefficient does not directly represent the volume of the substance, but rather the relative amount of molecules involved in the reaction.
Therefore, the correct way to describe the \(SO_3\)component in the reaction is option B: 8 molecules \(SO_3\). This represents the ratio of the number of molecules of \(SO_3\)that are produced in the reaction.
Option B
For more wsuch question on molecules visit:
https://brainly.com/question/475709
#SPJ8
A sample of nitrogen dioxide gas occupies 625 cm3 at 70.0 °C and 15.0 psi. What is the final Celsius temperature if the volume is 545 cm3 at 15.0 psi?
Answers
The final temperature of the sample of nitrogen dioxide gas that occupies 625 cm³ at 70.0 °C and 15.0 psi is 299.12K.
How to calculate temperature?The temperature of a gas can be calculated by using the combined gas law formula as follows:
P₁V₁/T₁ = P₂V₂/T₂
Where;
P₁ = initial pressureV₁ = initial volumeT₁ = initial temperatureP₂ = final pressureV₂ = final volumeT₂ = final temperatureAccording to this question, a sample of nitrogen dioxide gas occupies 625cm³ at 70.0 °C and 15.0 psi. The final temperature can be calculated as follows:
15 × 625/343 = 15 × 545/T
27.33T = 8175
T = 299.12K
Therefore, 299.12K is the final temperature of the sample of nitrogen dioxide gas.
Learn more about temperature at: https://brainly.com/question/18875024
#SPJ1
8. Does hydrogen have more electrons than lithium?
Answers
Answer:
Lithium has more electrons than Hydrogen
Explanation:
Lithium has 2,1 electrons
Hydrogen has 1 electrons
Does any one know how to do this

Answers
number 1 is...
Explanation:
B (i think)
75 POINTS!!!
Describe the plate movements in a Divergent(Constructive), Convergent (Destructive) and a Transform (Conservative) Plate Margin. (these are also called plate boundaries). Your answer should define these THREE types of margins or boundaries by explaining the type of movement that occurs.
Answers
The type of movement that occurs in the plate movement listed above include the following:
A divergent boundary occurs when two tectonic plates move away from each other.A convergent boundary occurs when lithospheric plates are moving towards one another.Transform boundaries are created when tectonic plates slide past each other horizontally.What is a Tectonic plate?These are gigantic pieces of the Earth's crust and uppermost mantle and are made up of oceanic crust and continental crust.
A convergent boundary as the name implies occurs when lithospheric plates are moving towards one another.
Read more about Tectonic plate here https://brainly.com/question/1162125
#SPJ1
? January 5/6. What can you do differently for
the last half of the year in science? Whether it is
improving study skills, turning work in on time,
etc
Answers
Answer:
Explanation:
I'd say art
How many different genus groups are there? List them
Answers

A 15.0 L rigid container was charged with 0.5 atm of Krypton gas and 1.5 atm of chlorine gas at 350 C. The krypton and chlorine react to form tetrachloride. What mass of krypton tetrachloride can be produced assuming 100% yield?
Answers
The mass of krypton tetrachloride that can be produced assuming 100% yield is mathematically given as
molar mass=33.29g
What mass of krypton tetrachloride can be produced assuming 100% yield?Generally, the equation for ideal gas is mathematically given as
PV=nRT
Therefore
n=(0.50)(15.)/0.082*623
n=0.147mol
Hence for clorine
n=0.441mol
Given the reaction
Kr+2cl2---->KrCL4
Hence
molar mass=225.60*0.147
molar mass=33.29g
Read more about Chemical Reaction
https://brainly.com/question/11231920
#SPJ1
A sample of ideal gas at room temperature occupies a volume of 29.0 L at a pressure of 342 torr . If the pressure changes to 1710 torr , with no change in the temperature or moles of gas, what is the new volume, V2 ? Express your answer with the appropriate units.
Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. To solve such, we need to know the relation between rate of effusion and molar mass of gases. Therefore, the new volume V₂ is 18L.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature. an ideal gas on the walls of the container is inversely proportional to the volume occupied that gas.
Mathematically, Boyle's law can be written as
P₁V₁ = P₂V₂
P₁=initial pressure=342 torr
P₂=final pressure=1710 torr
V₁ = initial volume= 29.0 L
V₂=final pressure=?
Substituting all the given values in the above equation, we get
V₂ = P₁V₁/P₂
V₂ = (342 × 29.0)/1710
V₂ = 18L
Therefore, the new volume V₂ is 18L.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ1
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
ldentify the number of moles of each element
present in one mole of NHạNO3.
# moles N:
# moles H:
#moles O:
Answers
2 moles N
2 moles H
3 moles O
Further explanationGiven
The compound is probably NH₂NO₃
Required
The number of moles
Solution
moles Nthere are 2 N in NH₂NO₃, so moles N = 2moles
moles Hthere are 2 H in NH₂NO₃, so moles H = 2moles
moles Othere are 3 O in NH₂NO₃, so moles O = 3 moles
Answer:
N=2 moles
H=4 moles
O=3 moles
Explanation: just did it on edg
In the laboratory, a general chemistry student measured the pH of a 0.592 M aqueous solution of triethanolamine, C6H15O3N to be 10.781. Use the information she obtained to determine the Kb for this base.
Kb(experiment) =_______
Answers
Answer:
\(Kb=6.16x10^{-7}\)
Explanation:
Hello,
In this case, given the pH of the base, we can compute the pOH as shown below:
\(pOH=14-pH=14-10.781=3.219\)
Next, we compute the concentration of the hydroxyl ions when the triethanolamine is dissociated:
\(pOH=-log([OH^-])\)
\([OH^-]=10^{-pOH}=10^{-3.219}=6.04x10^{-4}M\)
Then, by writing the equilibrium expression for the dissociation of triethanolamine we have:
\(Kb=\frac{[OH^-][C6H14O2N^+]}{[C6H15O3N ]}\)
That is suitable for the direct computation of Kb, knowing that based on the ICE procedure, \(x\) equals the concentration of hydroxyl ions that was previously, computed, therefore, we have:
\(Kb=\frac{6.04x10^{-4}M*6.04x10^{-4}M}{0.592M-6.04x10^{-4}M}\\ \\Kb=6.16x10^{-7}\)
Regards.
Boron control rods are used in nuclear reactors to absorb neutrons. What nuclide
forms when 1°B absorbs one neutron and produces an alpha particle?
Answers
Explanation:
living and nen living defrentAnswer:
11B
Explanation:
When 10B absorbs a neutron, it becomes the unstable isotope 11B, which quickly undergoes beta decay to form the stable isotope 11C. In this process, a neutron in the 10B nucleus is transformed into a proton, which increases the atomic number of the isotope from 5 to 6. The beta decay of 11B involves the emission of an electron (beta particle), which is produced when a neutron in the nucleus decays into a proton and an electron.
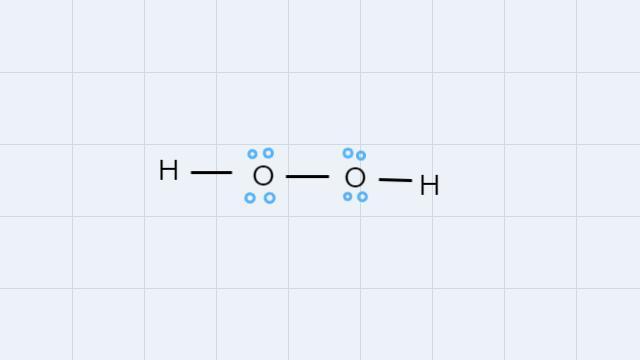
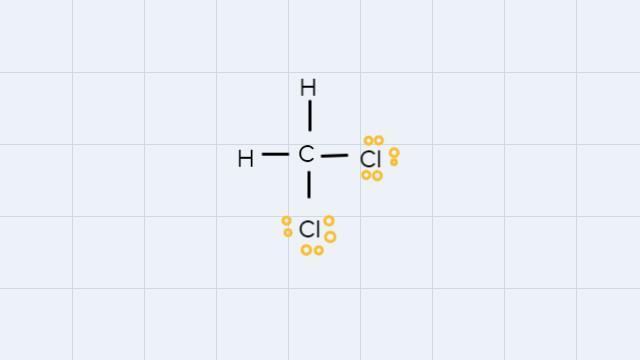
How to draw the lewis structures for the following? PCl3H2O2CH2Cl2H2CO2
Answers
Answer and explanation:
To draw the Lewis structure we have to make sure that each element in the molecule has 8 electrons (Octet rule) in the valence shell, or 2 electrons in the hydrogen atom.
• PCl3:
In the PCl3 molecule, each chlorine atom has an Octet with 8 electrons, and the phosphorus atom also has an Octet but it is expanded (that means, that P atomd can have more that 8 electrons). From period 3 of the Periodic Table, elements can expand their octet and have more than 8 electrons.
• H2O2:
In the H2O2 molecule, oxygen atoms have an Octet with 8 electrons and hydrogen atoms have an Octet with 2 electrons.
• CH2Cl2:
In the CH2Cl2 molecule, all atoms have octets with 8 electrons, except for hydrogen, which has an octet with 2 electrons.
• H2CO2:
In this case, the Octet rule is fulfilled and also for this, an oxygen atom must form a double bond with the chlorine atom.


The first five ionization energies of an unknown element are listed in the table above. which of the following statements correctly identifies the element and cites the evidence supporting the identification?
Answers
B) Al because the large difference between the third and forth ionization energies.
What is ionization energy?
Ionization energy (also called ionization potential) is the amount of energy required to remove an electron from an isolated atom or molecule in chemistry and physics. There is an ionization energy for each successively removed electron. However, the ionization energy associated with the removal of the first (most loosely held) electron is most commonly used.
The ionization energy of a chemical element, expressed in joules or electron volts, is usually measured in a discharge tube in which rapidly moving electrons produced by an electric current collide with gas atoms of the element, releasing one of them will be electronic.
B) Al because the large difference between the third and forth ionization energies.
To know more about ionization energies visit:
https://brainly.com/question/20658080
#SPJ4
A sample of the compound has 9 grams of cobalt. How many grams of fluorine does the sample have?
Answers
The mass (in grams) of fluorine the sample have is 2.91 g
How do I determine the mass of fluorine?First, we shall determine the mole of 9 grams of cobalt. Details below:
Mass of cobalt = 9 grams Molar mass of cobalt = 58.93 g/mol Mole of cobalt =?Mole = mass / molar mass
Mole of cobalt = 9 / 58.93
Mole of cobalt = 0.153 mole
Finally, we shall determine the mass of fluorine the present in the compound. Details below:
Molar mass of fluorine = 19 g/mol Mole of cobalt = 0.153 moleMole of fluorine = Mole of cobalt = 0.153 moleMass of fluorine = ?Mole = mass / molar mass
0.153 = Mass of fluorine / 19
Cross multiply
Mass of fluorine = 0.153 × 19
Mass of fluorine = 2.91 g
Thus, the mass of fluorine is 2.91 g
Learn more about mass:
https://brainly.com/question/21940152
#SPJ1
Compute the sugar content in an 8 oz sample of a soft drink. If the sugar content as per label on the product =10g per 100ml.
Answers
Answer:
\(m_{sugar}=23.7g\ sugar\)
Explanation:
Hello,
In this case, we can first compute the volume of the sample in mL from the ounces:
\(8oz*\frac{29.5735mL}{1oz} =236.6mL\)
Thus, with the volume of the sample, we can compute the amount of sugar given the 10 g of sugar per 100 mL of soft drink as shown below:
\(m_{sugar}=236.6mL*\frac{10g\ sugar}{100mL}\\ \\m_{sugar}=23.7g\ sugar\)
Best regards.
In which type of reaction spectator ions are absent?
a. Hydrolysis
b. Double Displacement
c. Displacement
d. Hydration
I'm confused between hydration and hydrolysis.
Answers
Reaction spectator ions are absent in displacement reaction
Spectator ion is an ion that exist as a reactant and a product in chemical reaction and a spectator ion do not participate in the reaction and present the same on both side of the reaction called spectator ion and the displacement reaction in which one element is replaces by another element in the compound and present the same at both side called as displacement and in that spectator ions are absent
Know more about reaction
https://brainly.com/question/14050476
#SPJ1
How much energy is required to raise the temperature of 4.0 g of mercury metal from 9.3 oC to 83.0 oC.
Answers
From the specific heat capacity of mercury, the amount of heat energy required to raise the temperature of 4.0 g of mercury metal from 9.3 °C to 83.0 °C is 77.792 J.
What is the specific capacity of mercury?The specific heat capacity of a substance is the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius or kelvin.
The specific heat capacity of a substance is a constant that can be used to calculate the amount of heat required to raise the temperature of a given mass of a substance to any temperature.
The specific heat capacity of mercury is 0.140 J/g/k.
The formula for calculating specific heat capacity is given below:
Specific heat capacity, c = Δq/mΔT
where;
Δq = heat change
m = mass of the substance
ΔT = temperature change
The Heat required, Δq, will then be:
Δq = m * c * ΔT
Heat required, Δq = 4.0 * 0.140 * (83.0 - 9.3)
Heat required, Δq = 77.792 J
Lear more about specific heat capacity at: https://brainly.com/question/26866234
#SPJ1
СH +
О, –
СО, +
НО
What is the balancing equation
Answers
Answer:
i would also like to know
Explanation:
plz..............................................
Which seasons in Atlanta GA have worst AQI
Answers
In Atlanta, GA, certain seasons are associated with poorer air quality due to various factors such as weather conditions, human activities, and geographical location.
Typically, the seasons with the worst AQI in Atlanta, GA, are summer and early fall. This is primarily due to the combination of high temperatures, stagnant air masses, and increased pollution from various sources.
During the summer months, Atlanta experiences hot and humid weather, which can contribute to the formation of ground-level ozone. Ozone is a harmful pollutant that is created when pollutants from vehicles, power plants, and industrial activities react with sunlight and heat. High levels of ozone can cause respiratory issues and other health problems.
In addition to ozone, Atlanta also experiences increased levels of particulate matter (PM) during the summer and early fall. PM refers to tiny particles suspended in the air, which can come from sources such as vehicle exhaust, industrial emissions, and wildfires.
These particles can be inhaled into the lungs and can have detrimental effects on respiratory health.
It's important to note that air quality can vary from year to year and is influenced by various factors. Local regulations, weather patterns, and changes in pollutant emissions can all impact the AQI during different seasons.
Monitoring air quality reports and taking necessary precautions such as reducing outdoor activities during times of poor air quality can help individuals stay informed and protect their health.
For more such question on air quality visit:
https://brainly.com/question/21173066
#SPJ8
4. Long answer type questions: a. b. C. d. e. f. g. h. j. i. What are the constituent gases of air? Why is the surrounding air not seen with the eyes? How do you prove that air supports burning? How do you show that air occupies space? How do you prove that air has weight? How is air useful to us? Mention any three points. Write any three properties of air. How can you say that air exerts force? Write any four effects of air pollution. Write any three causes of air pollution and any two control measures of it.
Answers
1. The constituent gases of air are:
Nitrogen Oxygen Argon Carbon Dioxide2. The surrounding air is not seen with the eyes because it is transparent. Air molecules are not visible to the na-ked eye, and they do not scatter or absorb visible light significantly. Therefore, air appears colorless and transparent.
What is air?3. To prove that air supports burning, you can perform an experiment with a burning candle. Place a glass jar or bell jar over a lit candle, ensuring that the jar is airtight. As the candle burns, it consumes oxygen from the air inside the jar. Eventually, the candle flame will go out due to the lack of oxygen, proving that air (specifically oxygen) is necessary for burning.
4. To show that air occupies space, you can perform a simple experiment using a plastic bottle or syringe. Fill the bottle or syringe with water, ensuring there are no air bubbles. Then, cover the opening tightly and try to compress the air inside. You will find that it is not possible to compress the air significantly, indicating that air occupies space.
5. To prove that air has weight, you can use a sensitive balance or scale. Weigh an airtight container or balloon, and then fill it with air. The weight of the container or balloon with the added air will be greater than its initial weight, demonstrating that air has weight.
6. Air is useful to us in various ways. Three points highlighting the importance of air are:
Breathing and RespirationCombustion and Energy ProductionClimate Regulation7. Three properties of air include:
Air is Compressible: Air can be compressed or expanded under different conditions, allowing it to fill various spaces and containers.Air has Mass: Air molecules have mass, which means air itself has weight. It exerts pressure on objects and surfaces.Air Exerts Pressure: Due to the collisions of air molecules with surfaces, air exerts pressure in all directions. This pressure is known as atmospheric pressure.Air exerts force in various ways. For example, air pressure allows objects like airplanes to fly by providing lift. Air resistance or drag opposes the motion of objects moving through the air, creating a force that can affect their speed and trajectory.
8. Four effects of air pollution include:
Respiratory ProblemsEnvironmental Damage:Climate ChangeHuman Health Impacts9. Causes of pollution:
Industrial EmissionsVehicle EmissionsResidential and Agricultural Activities10. Two control measures for air pollution include:
Emission ReductionAir Quality RegulationsLearn more about air on https://brainly.com/question/15215203
#SPJ1
Cumulative Exam Active
41 42 43 144
The electron configuration of nitrogen (N) is
O 1s²2s²2p³
O 1s²2s²2p4
O 1s²2s²2p5
O 1s²2s²2p6
Answers
The answer is: The electronic configuration of Nitrogen is \(1s^22s^22p^3\).
Electronic configuration: The electronic configuration is defined as the distribution of electrons of an atom in the atomic or molecular orbitals and is written using the labels for the subshell.
How to decide which orbital is filled first?
The order in which electrons are filled in atomic orbitals as:(Shown in image)
Just follow the arrows to select the orbitals, s orbital can have 2 electrons, p can have 6 electrons, d can have 10 electrons and f can 14 electrons.The electronic configuration in which the outer shell is completely filled is known as noble-gas configuration as they are similar to electronic configurations of noble gases.Now, the given element is nitrogen (\(N\)). The atomic number of Nitrogen is 7. Thus, these 7 electrons are filled as-\(1s^22s^22p^3\)
Therefore, the electronic configuration of Nitrogen is \(1s^22s^22p^3\).To learn more about the electronic configuration, visit:
https://brainly.com/question/21977349
#SPJ4

Nitrogen's complete electron configuration is 12s2s22p3.
The shorthand electron configuration for noble gases is [He] 2s22p3. Nitrogen has an atomic number of 7. The nitrogen atoms' nucleus contain this many protons. An atom that is neutral has an equal number of protons and electrons. Thus, the ground state electron configuration will consist of 7 electrons in the suitable s and p orbitals (state of lowest energy). For nitrogen, the entire electron configuration is 1s22s22p. Scientists may easily express and explain how the electrons are organized around the nitrogen atom's nucleus by using the configuration notation for nitrogen (N). As a result, it is simpler to comprehend and forecast how atoms will cooperate to form chemical bonds.
Learn more about electronic configuration here-
https://brainly.com/question/11309892
#SPJ9
according to the bohr model of an atom, what happens when an electron moves from the second energy level to the third energy level and then back to the second energy level?
Answers
Answer:
when the atom moves to the third energy level, its energy increases. However, when it goes back to the second energy level its overall energy decreases.
Explanation:
the smallest (or innermost) energy level has the least amount of energy and the largest (or outer most) level needs the most amount of energy. In order for the electron to move from one level to the other, it would need to match the energy of that level.
What is the blocks average speed nearest hundredth of a m/s
Answers
The question is incomplete, the complete question is;
A block is pulled 0.90 m to the right in 2.5 s. What is the blocks average speed to the nearest hundredth of a m/s
Answer:
0.36 m/s to the hundredth place
Explanation:
Now let us remember the definition of speed. Speed is defined in physics as distance/time.
Here we have the distance as 0.9 m
We have the time as 2.5 s
Hence the average speed is obtained from;
Speed = 0.9/2.5 = 0.36 m/s to the hundredth place
What would the volume of a gas be at -125°C, if it had a volume of 693 mL at 45°? A -1,925 mL b. 322.5 mL c. 2,279 mL d. 15.4 mL
Answers
Answer: b. 322.5 mL
Explanation:assume constant pressure, then
693/(273.15+45) = v/(273.15-125)
v= 693*148.15/318.15 = 322.7
By using Charles law, the volume will be 322.5 mL
What is Charle's law?
The volume of such an ideal gas is proportional to its absolute temperature at constant pressure, according to Charles' law.
According to Charles law.
\(T_{1} / T_{2} = V_{1} / V_{2}\)
where, T is temperature and V is volume.
What is volume?A substance or object's volume refers to the amount of 3D space it takes up.
Calculation of volume.
Given data:
V = 693 mL
\(T_{1}\) =-125°C = (273-125) = 148 K
\(T_{2}\) = 45° = (273+45) = 318 K
Put the value of given data in Charles law formula.
148 K/ 318 K = V / 693 mL
V = 322.5 mL.
Therefore, by using Charles law the volume of a gas will be 322.5 mL.
To know more about Charles law.
https://brainly.com/question/16927784
#SPJ2
Need answer now! 50 points
Consider the reaction 2Al + 6HBr → 2AlBr3 + 3H2. If 8 moles of Al react with 8 moles of HBr, what is the limiting reactant?
A Al
B AlBr3
C H3
D HBr
Answers
Answer:D, HBr
Explanation:
Answer:
D. HBr because divide the moles of the substances given in the question by the co-efficient in the equation. Smallest quotient is the limiting reactant
A lab group made a copper amine stock solution with a concentration of 0.021 M. A group member transfers 8.19 mL of this solution to a 25 mL volumetric flask and dilutes to the line with water. What is the concentration of the resulting solution?
Answers
Answer:
Explanation:
To calculate the concentration of the resulting solution, we can use the dilution equation:
C1V1 = C2V2
where C1 and V1 are the initial concentration and volume of the stock solution, and C2 and V2 are the final concentration and volume of the resulting solution.
Plugging in the given values, we get:
C1 = 0.021 M
V1 = 8.19 mL = 0.00819 L
V2 = 25 mL = 0.025 L
Solving for C2:
C2 = (C1 x V1) / V2
= (0.021 M x 0.00819 L) / 0.025 L
= 0.00687 M
Therefore, the concentration of the resulting solution is 0.00687 M.
To learn more :
https://brainly.in/question/2127115
A chemical reaction is shown below:
Glucose + Oxygen --> Water + Carbon Dioxide
How many grams of water would be produced if the reaction contained each of the following:
20 g of glucose
15 g of oxygen
30 g of carbon dioxide
SELECT AN ANSWER
65 g of water
15 g of water
35 g of water
5 g of water
Answers
Answer:
Mass = 8.46 g
Explanation:
Given data Mass of water produced = ? Mass of glucose = 20 g Mass of oxygen = 15 g
Solution:
Chemical equation:
C₆H₁₂O₆ + 6O₂ → 6H₂O + 6CO₂
Number of moles of glucose:
Number of moles = mass/molar mass
Number of moles = 20 g/ 180.16 g/mol
Number of moles = 0.11 mol
Number of moles of oxygen:
Number of moles = mass/molar mass
Number of moles = 15 g/ 32 g/mol
Number of moles = 0.47 mol
now we will compare the moles of water with oxygen and glucose.
C₆H₁₂O₆ : H₂O
1 : 6
0.11 : 6/1×0.11 = 0.66
O₂ : H₂O
6 : 6
0.47 : 0.47
Less number of moles of water are produced by oxygen thus it will limit the yield of water and act as limiting reactant.
Mass of water produced:
Mass = number of moles × molar mass
Mass = 0.47 mol ×18 g/mol
Mass = 8.46 g
Explanation: