A compound is determined to have the empirical formula C2OH4. If the molar mass of the compound is 132 g/mol, determine the molecular formula of the compound.

Answers
Answer: The molecular formula will be \(C_6O_3H_{12}\)
Explanation:
Molecular formula is the chemical formula which depicts the actual number of atoms of each element present in the compound.
Empirical formula is the simplest chemical formula which depicts the whole number of atoms of each element present in the compound.
Empirical weight of \(C_2OH_{4}\) is \(12\times 2+16\times 1+1\times 4=44g\)
Molecular mass of compound is = 132 g
Now we have to calculate the molecular formula.
\(n=\frac{\text{Molecular weight}}{\text{Equivalent weight}}=\frac{132}{44}=3\)
The molecular formula will be=\(3\times C_2OH_4=C_6O_3H_{12}\)
Related Questions
Generally, which element of film sparks the initial interest of a movie audience?
Answers
Despite what is portrayed in science fiction movies, if you witnessed a battle in outer space, you would not hear sound. Explain why.
Answers
Answer:
The vacuum of outer space has essentially zero air. Because sound is just vibrating air, space has no air to vibrate and therefore no sound.
Explanation:
The vacuum of outer space has essentially zero air. Because sound is just vibrating air, space has no air to vibrate and therefore no sound.
Answer:
There is no air in space for sound waves to travel
25. The two major signs of a scam are a request for personal information
and
Answers
Scammers often attempt to obtain personal information such as social security numbers, bank account details, or passwords, under the guise of legitimate organizations. scammers often make enticing promises that are unrealistic or too good to be true. These promises may include guaranteed high returns on investments, lottery winnings, or extravagant rewards for minimal effort.
The two major signs of a scam are a request for personal information and promises that seem too good to be true. Scammers often attempt to obtain personal information such as social security numbers, bank account details, or passwords, under the guise of legitimate organizations. They may use tactics like phishing emails, fake websites, or phone calls to deceive individuals into revealing sensitive information. It's important to remember that reputable organizations typically do not ask for personal information via unsolicited communication. Additionally, scammers often make enticing promises that are unrealistic or too good to be true. These promises may include guaranteed high returns on investments, lottery winnings, or extravagant rewards for minimal effort. Such offers are designed to lure unsuspecting individuals into providing money or personal information. Being cautious and skeptical, avoiding sharing personal information without verifying the legitimacy of the request, and conducting thorough research can help protect against falling victim to scams.
For more question on organizations
https://brainly.com/question/21011563
#SPJ11
Which is not a chemical element needed to build sugars?
Answers
Answer:
Nitrogen
Explanation:
Triangle DEF is congruent to TriangleD'EF' by the SSS theorem. Which single rigid transformation is required to map TriangleDEF onto TriangleD'EF'? dilation reflection rotation translation
Answers
Answer:
B. reflection
Explanation:
Rigid transformations are methods involved in changing the dimensions or orientation of a given figure. The methods are; dilation, rotation, translation and reflection.
Reflection is the process in which a given figure is turned or flipped with respect to a point or line of reference.
In the given question,
ΔDEF ≅ ΔD'EF' (Side-Side-Side congruence property)
This implies that ΔDEF was reflected about point E to produce ΔD'EF'. Thus the required rigid transformation is reflection.
Answer:
Is C - Rotation
Explanation:
I have right on my test
hexane followed by dichloromethane is used to separate the fluorene and 9-fluorenone. why would reversing the order in which these solvents are used be unwise?
Answers
9-fluorenone will get strongly bonded to the stationary phase.
9-fluorenone is comparatively more polar than fluorene because it contain a carbonyl group. We know that the stationary phase is alumina and alumina is a polar adsorbent. Alumina is a best choice for the separation of components that contain a weakly or moderately polar component. This kind of polar components of the mixture are retained for a longer time and more selectively by the adsorbent.
The fluorene is eluted first with the methane, this elution makes it easier to elute the 9-fluorenone with the dichloromethane. The 9-fluorenone will undergo slow elution because it is more strongly attracted to the alumina owing to its polar carbonyl group.
Fluorene moves faster because it's polarity is the same as methane and also opposite of the alumina. If the order of solvents is reversed and dichloromethane is used first, than the 9-fluorenone binds more strongly to the alumina making it difficult to elute fluorene using methane.
Learn more about elution from the link given below.
https://brainly.com/question/9630611
#SPJ4
How many grams of magnesium hydroxide will precipitate if 25. 0 mL of 0. 235 M magnesium nitrate are combined with 30. 0 mL of 0. 260 M potassium hydroxide?
Answers
The grams of magnesium hydroxide will precipitate if the 25 mL of 0.235 M magnesium nitrate are combined with the 30. 0 mL of 0.260 M potassium hydroxide is 0.227 g.
The reaction is given as :
Mg(NO₃)₂ + 2KOH ----> 2KNO₃(aq) + Mg(OH)₂(s)
moles of Mg(NO₃)₂ = 0.235 × 0.025
= 0.00587 mol
moles of KOH = 0.260 × 0.030
= 0.0078 mol
1 mole of Mg(NO₃)₂ react with 2 mole of KOH
mole of KOH = 0.0078 × 2
= 0.0156 mol
KOH is the limiting reagent.
2 mole of KOH produces 1 mole of Mg(OH)₂
mole of Mg(OH)₂ = 0.0078 / 2 = 0.0039 mol
mass of Mg(OH)₂ = 0.0039 × 58.3
= 0.227 g
To learn more about moles here
https://brainly.com/question/26416088
#SPJ4
The equation below shows the decomposition of lead nitrate. How many grams of oxygen are produced when 10.5 g NO2 is formed?
2Pb(NO3)2 (s) 2PbO (s) + 4NO2 (g) + O2 (g)
Answers
The mass of the oxygen that would be produced would be 1.84 g
What is the stoichiometry?
Stoichiometry calculations involve converting between mass, moles, and other units of substances using molar masses and stoichiometric ratios.
We know that;
Number of moles of nitrogen dioxide = 10.5 g/46 g/mol
= 0.23 moles
Now we have that;
4 moles of nitrogen dioxide is produced when 1 mole of oxygen is produced
0.23 moles of nitrogen dioxide would produce 0.23 * 1/4
= 0.0575 moles
Mass of the oxygen = 0.0575 moles * 32 g/mol
= 1.84 g
Learn more about stoichiometry:https://brainly.com/question/28780091
#SPJ1
which cycle results in death of the host cell
A.lytic cycle
B.lysogenic cycle
C.both
Answers
Answer:
A. lytic cycle is the correct answer
Scientists use bacteria to make medicines,
o True
o False
Answers
Answer:
true they do I yes they do
During a science class investigation, a teacher places a small amount of sugar in a beaker and then heats it over a candle. Which of the following would be the best evidence that a change in the chemical properties of the sugar has taken place?
Answers
The correct option is D. A gas is released and a black solid forms.
What proof is there that the burning of sugar involves a chemical reaction?Carbon, hydrogen, and oxygen atoms make up sugar. When heated over a candle, these materials react with the fire and turn into liquids. Heat causes the sugar's atoms to interact with the oxygen in the air, forming new atomic groups. Energy is released during this chemical reaction in the form of smoke and black soot.
What happens chemically when sugar is consumed?The main functions of sugars include their ability to sweeten things, maintain and intensify flavours, act as antioxidants and preservatives, and interact with water to change how it behaves. Fermentation, caramelization, Maillard reactions, or the creation of browning compounds, are examples of chemical processes.
To know more about solid visit:-
https://brainly.com/question/17061172
#SPJ1
Question:
During a science class investigation, a teacher places a small amount of sugar in a beaker and then heats it over a candle. Which of the following would be the best evidence that a change in the chemical properties of the sugar has taken place?
A. The sugar changes color.
B. The sugar dissolves in the water.
C. The sugar melts and turns into a liquid.
D. A gas is released and a black solid forms.
What could happen if the chemists do not follow the exact formula for medications? (Give examples)
Answers
Answer:
qwe
Explanation:
wqe
About 10% of the water enters the atmosphere through
Answers
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
Why would scientists use molar volume to measure gases?
Answers
Answer:
The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions.
scientists use molar volume to measure gas because it it tells us about volume occupied by gas.
Molar volume
The molar volume of a gas tell us about volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions.At STP(Standard Temperature and Pressure) condition which is equal to 22.4 L for 1 mole of any ideal gas at a temperature equal to 273.15 K and a pressure equal to 1.00 atm.The molar volume of a gas is derived from the ideal gas law PV=NRT
What is the maximum number of orbitals that can be identified with the following quantum numbers?
n=3,l=+1,m l =0
a. 1
b. 2
c. 3
d. 4
Answers
The maximum number of orbitals that can be identified with the given quantum numbers is 2.
The quantum number n=3 indicates that the shell or energy level of the atom is the third level. The quantum number l=+1 (or l=1) indicates that the subshell is p-type. The quantum number ml=0 indicates that the orientation of the orbital is along the z-axis. For a p-type subshell, there are three possible orientations: ml=-1, 0, and +1. Therefore, there are three orbitals possible for the given quantum numbers.
However, the quantum number ml=0 specifies only one of these three orbitals, leaving two possible orbitals for the other two values of ml. Hence, the maximum number of orbitals that can be identified with the given quantum numbers is 2. Therefore, the correct answer is b. 2.
For more such question related to quantum number : https://brainly.com/question/2292596
#SPJ11
what is the formula for radiant to chemical energy to make glucose
Answers
The radiant energy that is absorbed by the plant in the process of photosynthesis is stored in the plant in the form of chemical energy contained in sugars.
What is photosynthesis?The term photosynthesis has to do with the process by which there is a combination of water and carbon dioxide in the plant such that the products that are formed are sugar and oxygen. The oxygen is released back into the environment.
We know that radiant energy from the sun is what acts as the catalyst that makes the reaction possible. The combination occurs in the chloroplasts of the plant cells .
Learn more about photosynthesis:https://brainly.com/question/1388366
#SPJ1
Consider the following pair of reactions. Predict the type of substitution mechanism, predict which reaction of the pair will occur at the faster rate, and draw the correct organic product
Answers
The reaction with S_N₂mechanism is likely to be faster than the reaction with S_N₂ mechanism. This is because the carbocation intermediate formed in S_N₁ mechanism is more stable.
The pair of reactions given below is:
CH₃Cl + NaOH→CH₃OH + NaCl
CH₃I + NaOH→CH₃OH + NaI
The type of substitution mechanism:
The first reaction involves S_N₁ mechanism (unimolecular nucleophilic substitution). The second reaction involves S_N₂ mechanism (bimolecular nucleophilic substitution).
Prediction of the reaction that will occur at a faster rate:
The reaction with S_N₁ mechanism is likely to be faster. The rate of this reaction mainly depends on the stability of the carbocation intermediate formed after the initial step. In this case,CH₃Cl reacts to form a tertiary carbocation which is more stable than the primary carbocation formed in CH₃I.
Drawing the correct organic product:
CH₃Cl + NaOH→CH₃OH + NaCl
CH₃I + NaOH→CH_3OH + NaI
CH₃C reacts with NaOHin an S_N₁ mechanism to produceCH₃OH and NaCl.
CH₃ reacts withNaOH in an S_N₂mechanism to produce CH₃OH and NaCI.
To know more about unimolecular nucleophilic substitution visit:
brainly.com/question/32657850
#SPJ11
How many grams are there in 1.45 kg?
Answers
Answer:
1.45 kg
Explanation:
common sense ok
Answer:
1450 grams are in 1.45 kilograms
Hammerhead sharks have weakly-muscled gills and must be in constant motion in the ocean in order to maintain a steady flow of water over their gills. The steady flow of water across their gills is necessary for –
attracting mates.
staying warm.
attacking prey.
obtaining oxygen.
Answers
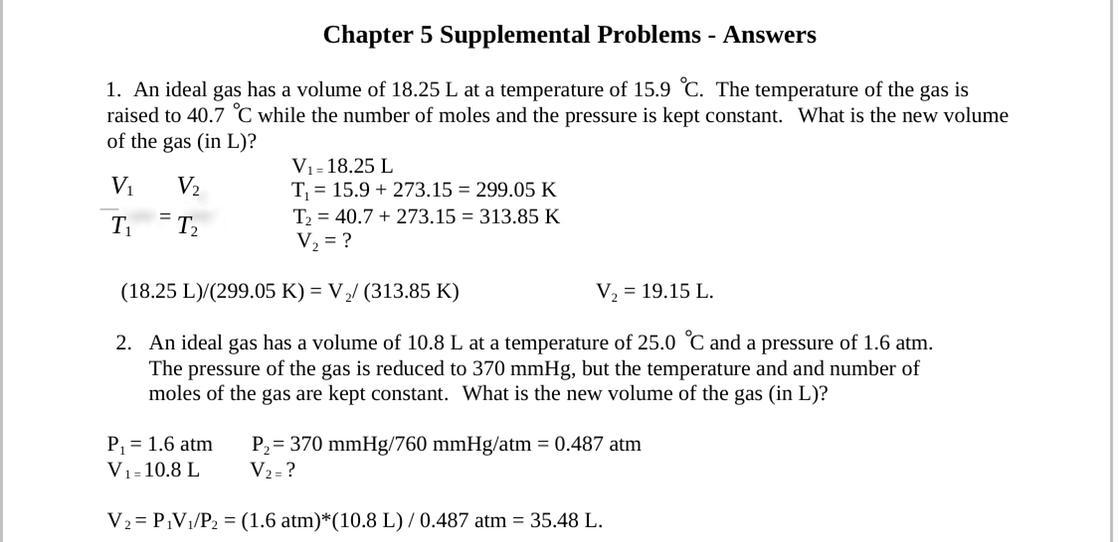
An ideal gas has a volume of 18.25 L at a temperature of 15.9 °C. The temperature of the gas is raised to 40.7 °C while the number of moles and the pressure of the gas are kept constant. What is the new volume of the gas (in L)?
B: An ideal gas has a volume of 10.8 L at a temperature of 25.0 °C and a pressure of 60 atm. The pressure of the gas is reduced to 370.0 mmHg, but the temperature and number of moles of the gas are kept constant. What is the new volume of the gas (in L)?
Answers
Answer:
V₂ = V₁ / T₁ * T₂ . If you prefer to set the final volume and want to estimate the resulting temperature, then the equation of Charles' law changes to: T2=T1/T1 multiplied by v^2.
The activation energy for the reaction between zinc and sulfuric acid is lowered if a solution containing
metal ions is added.
What is the most likely formula of the metal ions added?
Al3+
Ca²+
Cu²+
Na+
Answers
Copper ions would increase the activation energy. So the correct option is C.
What is activation energy?
The least amount of energy necessary to bring atoms or molecules into a state where they may cause chemical modification or physical transport is known as the activation energy in chemistry.
The differential in total energy between atoms or molecules in an energized or transition-state structure and the same atoms and molecules in their original configuration is known as the activation energy in transition-state theory.
Experimental rate constants or distribution coefficients that are measured at various temperatures are used to calculate activation energies.
Therefore the correct option is C.
Read more about activation energy, here
https://brainly.com/question/28384644
#SPJ1
When light energy hits matter, energy is formed. A. Heat B. Potentail C. Mechanical
Answers
Heat energy is formed.
Law of Conservation of Energy, states that energy can neither be created nor destroyed; energy can only be transferred or changed from one form to another.
Describe conversion of light energy to heat energy.
According to above question, light is a form of energy ,therefore can be converted to electricity with the help of solar cell , that electricity can be used to drive a mechanical motor or engine. Thus, it can be converted to form heat energy.
Another example of light energy converting into heat energy is when sunlight hits an object, it can be reflected or absorbed. If it is reflected it bounces off at the same wavelength. But if it is absorbed, the short wavelength energy is changed to long wavelength (heat).
To know more about conservation of energy, click on https://brainly.com/question/166559
#SPJ4
What do we call matter made you of only one kind of atom?
And Atoms have weight and take up space
Answers
Answer:
Matter is any substance, if it is made up of only one kind of atom, then it is an element. Elements consists of atoms with the same weight and same number of protons in the nuclei.
A woman and her daughter were found dead at their summer cabin with gunshots to the head. Her husband claims to have found a note saying his wife killed their daughter and then planned to kill herself. The blood spatter expert brought in to investigate didn’t believe his story because the pattern of the blood was inconsistent with what?
Group of answer choices
his wife’s blood type
the type of gun she was holding
the time of day he said the incident happened
a self-inflicted wound
Answers
I will say 3 is wrong because the time of day doesn't matter.But what really matters is 1,2,4 we just need to figure which one is correct.
1.The Wife's blood
Real blood will smell bad and with the time it will turn the color red to a dark-ish color red with brown.To see if it is real you have to see if the blood is a dark brown with red if it's not then it's a fake.
2.The type of Gun
You have to feel if it's a fake it might be plastic or another toy material.The gun has to feel like metal.
So the answer might be 1 or 2
Hope this helps :)
piece of copper iron silver and gold are dropped into a solution of iron sulphate the piece that will get a coating of a copper is known as
Answers
Pieces of copper, silver and gold are dropped into a solution of iron sulphate. The piece that will get a coating of copper is. ... Therefore, none of the three metals can displace iron from its salt solution. Hence, we observe that no reaction takes place and none of the pieces get coated.
Answer:
If it were a piece of iron dropped into a copper sulfate solution, precipitated copper would coat the iron
C3H5N3O9(s) --> N2(g) + CO2(g) + H2O(g) + O2(g) Solid nitroglycerine explodes when heated, producing several different gases (according to the reaction above). If a government scientist explodes 777 g of nitroglycerine, How many grams of each gas should be produced? How many molecules of each gas will be produced?
Answers
Answer:
- 143.6 g of N₂ ; 3.08×10²⁴ molecules of N₂
- 451.4 g of CO₂ ; 6.17×10²⁴ molecules of CO₂
- 153.9 g of H₂O ; 5.14×10²⁴ molecules of H₂O
- 27.3 g of O₂ ; 5.14×10²³ molecules of O₂
Explanation:
This is a reaction of decomposition:
4C₃H₅N₃O₉(s) → 6N₂(g) + 12CO₂(g) + 10H₂O(g) + O₂(g)
4 moles of solid nitroglycerine decompose to 6 moles of nitrogen, 12 moles of carbon dioxide, 10 moles of water vapor and 1 mol of oxygen
We convert mass to moles → 777 g. 1mol/ 227g = 3.42 moles
4 moles of C₃H₅N₃O₉ can decompose to:
6 moles of N₂ ____ 12 moles of CO₂ ___ 10 moles of H₂O ___ 1 mol of O₂
Then, 3.42 moles of C₃H₅N₃O₉ may decompose to:
(3.42 . 6) / 4 = 5.13 moles of N₂
(3.42 . 12) / 4 = 10.26 moles of CO₂
(3.42 . 10) / 4 = 8.55 moles of water vapor
(3.42 . 1) / 4 = 0.855 moles of oxygen
We convert the moles to mass:
5.13 mol . 28 g/mol = 143.6 g of N₂
10.26 mol . 44 g/mol = 451.4 g of CO₂
8.55 mol . 18 g/mol = 153.9 g of H₂O
0.855 mol . 28 g/mol = 27.3 g of O₂
We count the atoms:
5.13 mol . 6.02×10²³ molecules /mol = 3.08×10²⁴ molecules of N₂
10.26 mol . 6.02×10²³ molecules /mol = 6.17×10²⁴ molecules of CO₂
8.55 mol . 6.02×10²³ molecules /mol = 5.14×10²⁴ molecules of H₂O
0.855 mol . 6.02×10²³ molecules /mol = 5.14×10²³ molecules of O₂
some reactions can be performed without a solvent. what are the benefits of not needing a solvent in a reaction? select one or more: the reaction often costs less because solvents can be expensive. less chemical waste is generated because there are not solvents to remove. reaction progress is easy to monitor because the reagents are more concentrated. the reaction rate is smaller because the concentration of reagents is greater.
Answers
Less chemical waste is generated because there are no solvents to remove. The reaction often costs less because solvents can be expensive.
The reaction rate is greater because the concentration of reagents is greater. These options are correct.
A solvent is a substance that dissolves a solute, producing a solution. In addition to being a liquid, a supercritical fluid, a solid, or a gas can also be solvent. All the ions and proteins in a cell are dissolved in water, which is a solvent for polar molecules and the most frequent solvent employed by living things.
chemical reaction, the transformation of one or more chemicals (the reactants) into one or more distinct compounds (the products). Chemical elements or chemical compounds make up substances. In a chemical reaction, the atoms that make up the reactants are rearranged to produce various products.
Learn more about Solvent here: brainly.com/question/30885015
#SPJ4
Daffodil plant: What's the Difference between small and medium???
Answers
Answer:
Daffodils are the most carefree of all spring flowers. ... Daffodil flowers come in many different shapes, sizes, and color combinations. The American Daffodil Society classifies daffodils by their flower shape, and there are officially 13 different classifications.
Explanation:
the small one: they grow from fall-planted bulbs and bloom in spring. The size of a dwarf daffodil depends on the variety, but generally, they grow 4 to 6 inches (10 to 15 cm.)
and i can't find the medium size, sorry
hope it helps
Describe and give the results of a test to show that sodium hydroxide is a stronger alkali than ammonia solution.
Answers
Because it completely dissociates into hydroxide ions when placed in water, sodium hydroxide (NaOH) is a strong basic.
While producing less hydroxide ions in solution by accepting protons from water, ammonia (NH3) is a weak base.
What distinguishes sodium hydroxide from ammonia?The inorganic substances sodium hydroxide and ammonium hydroxide are distinct from one another. Sodium hydroxide is a metal hydroxide with the chemical formula NaOH, whereas ammonium hydroxide is a liquid with the chemical formula NH4OH.
Because it entirely dissociates into sodium cation and hydroxide anion in aqueous solution, sodium hydroxide is a strong basic. One mole of sodium hydroxide will thus include one mole of salt composed and one mole of hydroxide anion as one formula unit of sodium hydroxide comprises one salt cation and one hydroxide anion.
Learn more about sodium hydroxide refer
https://brainly.com/question/25597694
#SPJ10