Answers
In order to calculate the number of grams of magnesium reacted, we must first calculate the moles of hydrogen gas produced. The ideal gas law can be used to calculate the moles of a gas, given the pressure, temperature, and volume of the gas.
The ideal gas law is PV=nRT, where P is pressure, V is volume, n is moles, R is the ideal gas constant, and T is temperature. By calculating the given values, we get 0.032L*1.1atm/24C*0.08206L*atm/mol*K=0.0077 mol.
Since 1 mole of hydrogen gas is produced for every mole of magnesium that reacts, 0.0077 moles of magnesium have reacted.
Finally, to calculate the grams of magnesium, we multiply the moles of magnesium by the molar mass of magnesium, which is 24.31 g/mol. This gives us 0.0077 mol*24.31 g/mol = 0.186 grams of magnesium.
Learn more about magnesium at :
https://brainly.com/question/1533548
#SPJ1
Related Questions
Help fast plz!!!! 2. Which is larger, a gene or DNA?
A gene is larger
DNA is larger
Answers
How many carbon atoms are there in 5 molecules of C6H12O6?
Answers
Answer:
6
Explanation:
Glucose has a chemical formula of: C6H12O6 That means glucose is made of 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms.
What energy transformations occur in a hot air balloon?
Answers
Answer:
: kinetic energy is the energy transformation that occurs in a hot balloon.
Explanation:
Hot air balloons use a propane burner that converts chemical energy to thermal energy. The hot air is less dense than than the colder air and it lifts the balloon
How many grams of KOH are needed to neutralize 13.4 mL of 0.17 M HCl in stomach acid?
Answers
Answer:
0.1288g of KOH are needed.
Explanation:
1st) It is necessary to write and balance the chemical reaction:
\(KOH+HCl\rightarrow KCl+H_2O\)From the balanced reaction we know that 1 mole of KOH neutralizes 1 mole of HCl.
2nd) We have to calculate the number of HCl moles that are contained in 13.4mL of a 0.17M solution.
The molarity of the solution indicates that there are 0.17 moles of HCl in 1000mL of solution, so we can calculate the number of moles in 13.4mL of solution, using a mathematical rule of three:
\(\begin{gathered} 1000mL-0.17molesHCl \\ 13.4mL-x=\frac{13.4mL*0.17molesHCl}{1000mL} \\ x=2.3*10^{-3}molesHCl \end{gathered}\)Now we know that there are 2.3x10^-3 moles of HCl in the solution.
3rd) From the stoichiometry of the reation, 1 mole of KOH neutralizes 1 mole of HCl. So, since the relation between KOH and HCl is 1:1, in this case, 2.3x10-3 moles of KOH are needed to neutralize the HCl.
Using the molar mass of KOH (56g/mol) we can convert the moles into grams:
\(2.3*10^{-3}moles*\frac{56g}{1mole}=0.1288g\)Finally, 0.1288g of KOH are needed.
prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8
if a runner decreases his velocity from +20 m/s to +10 m/s what is his average acceleration
Answers
Answer:
f a runner decreases his velocity from +20 m/s to +10 m/s, with average acceleration is -5m/s².
Explanation:
5.96 g of ammonia reacts completely according to the following reaction:
2 NH, (g) + Co, (g) → CN,OH, (s) + H20 (1)
(a) What is the theoretical yield of urea (CN,OH,) for this reaction?
(b) If 13.74 g of urea are produced, what is the percent yield for this equation?
please show work, will give brainliest
Answers
Explanation:
this explanation may help u to understand:)

What is O?
(A)Metal
(B)Metalloid
(C)Nonmetal
(D)None of the above
Answers
O, representing oxygen on the periodic table, is a nonmetal.
Answer:nonmetal
Explanation: gggg
1. What is the modern view of electrons in the quantum mechanical model?
Answers
Answer: An electron con only exist in a limited number of quantized energy levels.
Explanation:
Help me please) attached the screen below. Thanks

Answers
Answer:
1) d = 2.4 g/cm³
2) m = 25 g
3) v = 126.7 cm³
Explanation:
Given data:
Mass of material = 24 g
Volume of material = 10 cm³
Density of material = ?
Solution:
Formula:
d = m/v
by putting value,
d = 24 g / 10 cm³
d = 2.4 g/cm³
2) Given data:
Density of material = 5 g/cm³
Volume of material = 5 cm³
Mass of material = ?
Solution:
Formula:
d = m/v
5 g/cm³ = m / 5 cm³
m = 5 g/cm³×5 cm³
m = 25 g
3)Given data:
Density of material = 3 g/cm³
Mass of material = 380 g
Volume of material = ?
Solution:
Formula:
d = m/v
3 g/cm³ = 380 g / v
v = 380 g /3 g/cm³
v = 126.7 cm³
You walk into the lab, and you find a beaker sitting on the bench labeled HNO3. However, the concentration is not given. Your instructor tells you to do a titration to determine the concentration of the acid. You find that is takes 27.60 mL of 1.00 M NaOH to neutralize 10.00 of the HNO3. What is the concentration oft the HNO3?
HNO3 + NaOH
H2O + NaNO3
Answers
The concentration of the HNO₃ solution needed to neutralize the 27.60 mL of 1.00 M NaOH is 2.76 M
How do i determine the concentration of the HNO₃ solution?The balanced equtaion is given below:
HNO₃ + NaOH —> H₂O + NaNO₃
Mole ratio of the HNO₃ (nA) = 1Mole ratio of the NaOH (nB) = 1Now, we shall obtain the concentration of the HNO₃ solution needed for the neutralization reaction. This is shown below:
Volume of HNO₃ (Va) = 10 mLVolume of NaOH (Vb) = 27.60 mLConcentration of NaOH (Cb) = 1.00 M Concentration of HNO₃ (Ca) =?CaVa / CbVb = nA / nB
(Ca × 10) / (1 × 27.6) = 1
(Ca × 10) / 27.6 = 1
Cross multiply
Ca × 10 = 27.6
Divide both side by 10
Ca = 27.6 / 10
Ca = 2.76 M
Thus, the concentration of the HNO₃ solution needed is 2.76 M
Learn more about titration:
https://brainly.com/question/27817549
#SPJ1
A gas mixture contains 3.50 moles of helium, 5.00 moles of krypton and 7.60 moles of neon. A) What is the mole fraction for each gas
Answers
Answer:
.217, .311, and .472, respectively.
Explanation:
The total number of moles of gas is 3.50 + 5.00 + 7.60 = 16.10 (to preserve significant digits).
X of helium=3.50/16.10 = .217
X of krypton=5.00/16.10 = .311
X of neon=7.60/16.10 = .472
which animal takes on the temperature of their surroundings and does not use food energy to keep warm
Answers
What’s the answer to this?
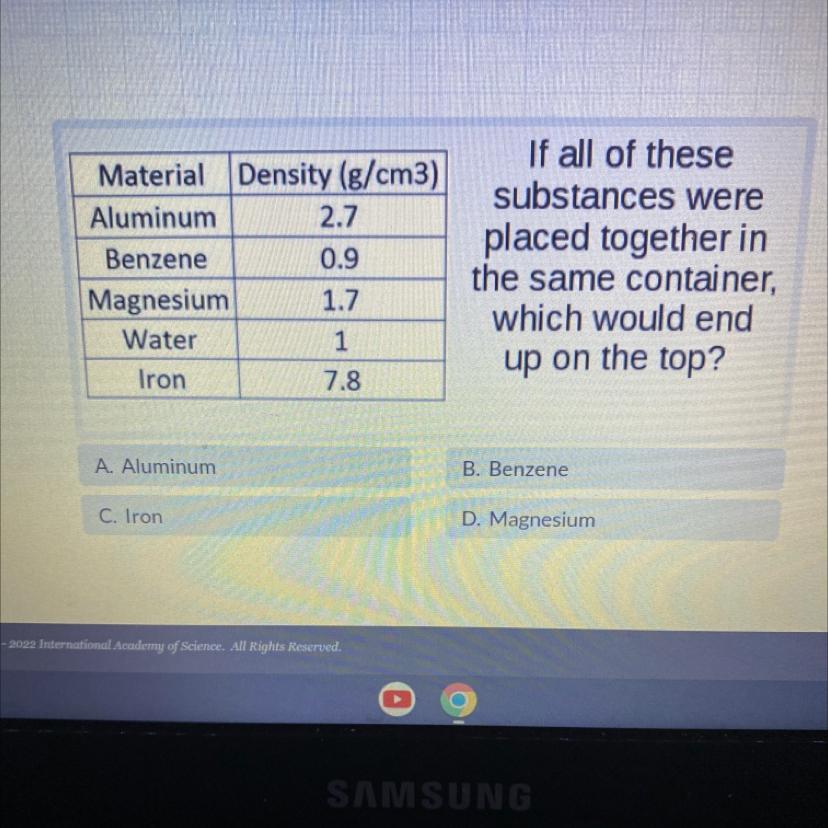
Answers
Benzene
Benzene has low density than the water itself of about 0.9 g/cm³
Low denisty object than the water usually floats on the water.
Another example: orange peel which floats on water has density of about 0.84 g/cm³
CHEM FINAL TOMORROW!!! I'm struggling with a few concepts, if anyone could help explain this to me & how to do it, I'd be very grateful!!!

Answers
Based on the given reaction, the acid-base pairs in this reaction are:
HCO₃⁻ (acid) and NH₃ (base)NH₄⁺ (acid) and CO₃²⁻ (base)What are the acid-base pairs in the given reaction?An acid-base pair refers to a set of two chemical species that are related through the transfer of a proton (H+ ion) during a chemical reaction.
One species acts as an acid by donating a proton, while the other acts as a base by accepting that proton.
In the given reaction:
HCO₃⁻ (aq) + NH₃ (aq) → NH₄⁺ + CO₃²⁻
An acid-base pair can be identified as follows:
HCO₃⁻ (bicarbonate ion) can act as an acid by donating a proton (H⁺), becoming CO₃⁻.
NH₃ (ammonia) can act as a base by accepting a proton (H⁺), becoming NH₄⁺ (ammonium ion).
Learn more about acid-base pairs at: https://brainly.com/question/22514615
#SPJ1
Calculate the pH of a solution prepared by dissolving 1.60 g of sodium acetate, CH3COONa, in 50.0 mL of 0.10 M acetic acid, CH3COOH(aq). Assume the volume change upon dissolving the sodium acetate is negligible. Ka of CH3COOH is 1.75 x 10^-5.
Answers
Answer:
pH = 5.35
Explanation:
Given 1.60 grams sodium acetate (NaOAc(aq))*** added to 50ml of 0.10M acetic acid (HOAc(aq)) solution.
Applying common ion effect keeping in mind that the addition of NaOAc provides the common-ion (OAc⁻).
HOAc(aq) ⇄ H⁺(aq) + OAc⁻(aq)
I 0.10m 1.32 x 10⁻³M ≈ ∅M* (1.6g/82.03g/mol) / 0.050L = 0.39M
C -x +x 0.39M + x ≈ 0.39M**
E 0.10M - x x 0.39M
≈ 0.10M
Ka = [H⁺][OAC⁻]/[HOAC] => [H⁺] = Ka·[HOAc] / [OAc⁻]
[H⁺] = (1.75 X 10⁻⁵)(0.10) / (0.39) = 4.5 x 10⁻⁶M
∴ pH = -log[H⁺] = -log(4.5 x 10⁻⁶) = -(-5.35) = 5.35
_______________________________________________
* [H⁺] before adding NaOAc = SqrRt(Ka · [HOAc]) = SqrRt(1.75 x 10⁻⁵· 0.10) = 1.32 x 10⁻³M. Since this concentration value is so small, the initial [H⁺] is assumed to be zero molar (∅M).
** The added [H⁺] is negligible and dropped in the ICE table. That is, adding ~[H⁺] in the order of 10⁻³M does not change the H⁺ ion concentration sufficiently to affect problem outcome and is therefore dropped in the ICE table.
*** Acetic Acid and Sodium Acetate are frequently written HOAc and NaOAc where the OAc⁻ anion is the acetate ion (CH₃COO⁻) for brevity.
The pH of the solution measures the acid of the liquid throughout the molar concentration of hydrogen ions in a solution.
\(\bold{CH_3COONa \ mass = 1.60\ g}\\\\\)
Solution Volume (V) \(=50.0\ mL =0.05\ L\\\\\)
\(\bold{CH3COONa \ molarity =0.10\ M }\)
\(\bold{\text{Calculating the CH3COONa moles} =\frac{mass}{molar\ mass}}\)
\(= \frac{1.60\ g}{82.03\frac{g}{mol}} \\\\=0.0195\ mol\\\\\)
\(\bold{M = \frac{ \text{amount of solute moles} } { \text{solution volume in L} }}\)
\(= \frac{0.0195\ mol}{0.05\ L}\\\\ =0.39\ M\)
Using Henderson Hasselbach equation:
\(\bold{pH = -\log K_{a} + \log{[salt]}{[acid]}}\\\\\)
\(=\bold{ -\log (1.75\times 10^{-5}) + \log ( \frac{0.39}{0.10}) }\\\\=\bold{ 4.757 + \log (3.9)}\\\\=\bold{ 4.757 + 0.5910}\\\\=\bold{ 5.348}\\\\=\bold{ 5.35}\\\)
So, the final answer is "5.35".
Learn more:
brainly.com/question/1195974
please help!!! A, B, C, or D

Answers
Answer:
A
Refer to pic for explanation
Which statement is true of energy in reactants during an endothermic reaction?(1 point)
The energy found in the reactants remains in the system, and the reactants also take energy from the surroundings.
All of the energy from the reactants will be lost to the surroundings.
All of the energy from the reactants will remain in the system
Some of the energy in the reactants will remain in them after the reaction, but some is lost to the surroundings. plz anwres right for extra points
Answers
The energy found in the reactants remains in the system, and the reactants also take energy from the surroundings.
How many atoms of iron are in 3.47 g of iron?
Answers
Answer:
Explanation:
The atomic weight of iron (Fe) is 55.845 u. Since it's impossible to count the amount of atoms in a given mass of a substance, we measure atoms in moles. No matter what the element or compound is, there will be \(6.022 * 10^{23}\) atoms in one mole of compound. This is called Avogadro's number. Atomic weight, also called molar mass, is measured in grams per mole. We first find the number of moles of iron in 3.47 grams by setting up an inequality: \(\frac{55.845 g}{1 mol} = \frac{3.47 g}{x mol}\). Solving this inequality shows that there are 0.06213627 moles of iron in 3.47 grams. Now, all we do is multiply that value by Avogadro's number, \(6.022*10^{23}\). This gives us 3.7418 * 10^22 atoms in 3.47 grams of iron.
How many molecules of ethanol, C2H5OH, are contained in a 150. gram sample?
1.96 x 1024
46.0
6.02 x 1023
5.1 x 10-25
Answers
Answer:
1.96 × 10²⁴ molecules
Explanation:
Step 1: Given data
Mass of ethanol (m): 150. g
Step 2: Calculate the number of moles (n) corresponding to 150. g of ethanol
The molar mass of ethanol is 46.07 g/mol.
150. g × 1 mol/46.07 g = 3.26 mol
Step 3: Calculate the number of molecules in 3.26 moles of ethanol
To convert moles into molecules, we need Avogadro's number: there are 6.02 × 10²³ molecules in 1 mole of molecules.
3.26 mol × 6.02 × 10²³ molecules/1 mol = 1.96 × 10²⁴ molecules
A 250.0-mL flask contains 0.2500 g of a volatile oxide of nitrogen. The pressure in the flask is 760.0 mmHg at 17.00°C. How many moles of gas are in the flask?
Answers
Answer:
0.0104 moles of gas in the flask.
Explanation:
To calculate the number of moles of gas in the flask, you can use the ideal gas law equation: PV = nRT. Where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant and T is temperature.
First, you need to convert the pressure from mmHg to atm and the temperature from Celsius to Kelvin. The pressure in atm is 760.0 mmHg / 760 mmHg/atm = 1 atm. The temperature in Kelvin is 17.00°C + 273.15 = 290.15 K.
Next, you need to convert the volume from mL to L. The volume in L is 250.0 mL / 1000 mL/L = 0.2500 L.
Now you can plug all the values into the ideal gas law equation and solve for n: (1 atm)(0.2500 L) = n(0.08206 L·atm/mol·K)(290.15 K). Solving for n gives n = 0.0104 mol.
So there are approximately 0.0104 moles of gas in the flask.
Which animal is a primary consumer in the Ethiopian Highlands?
Answers
Answer:
I believe it is the Ethiopian ibex
Explanation:
Answer:
I think it the Ethiopian ibex
Explanation:
im srry if it wrong.
hope this helps tho
Inquiry Extension Consider a reaction that occurs between solid potassium and chlorine gas. If you start with an initial mass of 15.20 g K, and an initial mass of 2.830 g Cl2, calculate which reactant is limiting. Explain how to determine how much more of the limiting reactant would be needed to completely consume the excess reactant. Verify your explanation with an example
Answers
The 3.13 g of K would be needed to completely react with the remaining \(Cl_2\).
To determine which reactant is limiting, we need to calculate the amount of product that can be formed from each reactant and compare them. The reactant that produces less product is the limiting reactant, since the reaction cannot proceed further once it is consumed.
The balanced chemical equation for the reaction between solid potassium and chlorine gas is:
2 K(s) + \(Cl_2\)(g) -> 2 KCl(s)
From the equation, we can see that 2 moles of K react with 1 mole of \(Cl_2\) to form 2 moles of KCl.
First, we need to convert the masses of K and \(Cl_2\) into moles:
moles of K = 15.20 g / 39.10 g/mol = 0.388 mol
moles of \(Cl_2\) = 2.830 g / 70.90 g/mol = 0.040 mol
Now, we can use the mole ratio from the balanced equation to calculate the theoretical yield of KCl from each reactant:
Theoretical yield of KCl from K: 0.388 mol K x (2 mol KCl / 2 mol K) = 0.388 mol KCl
Theoretical yield of KCl from \(Cl_2\): 0.040 mol \(Cl_2\) x (2 mol KCl / 1 mol \(Cl_2\)) = 0.080 mol KCl
We can see that the theoretical yield of KCl from K is 0.388 mol, while the theoretical yield of KCl from \(Cl_2\) is 0.080 mol. Therefore, the limiting reactant is \(Cl_2\), since it produces less product.
To determine how much more of the limiting reactant would be needed to completely consume the excess reactant, we can use the stoichiometry of the balanced equation.
We know that 1 mole of \(Cl_2\) reacts with 2 moles of K to produce 2 moles of KCl. Therefore, the amount of additional K needed to react with the remaining \(Cl_2\) can be calculated as follows:
moles of K needed = 0.040 mol \(Cl_2\) x (2 mol K / 1 mol \(Cl_2\))
= 0.080 mol K
This means that 0.080 moles of K would be needed to completely consume the remaining \(Cl_2\). We can convert this to a mass by multiplying by the molar mass of K:
mass of K needed = 0.080 mol K x 39.10 g/mol
= 3.13 g K
Therefore, The 3.13 g of K would be needed to completely react with the remaining.
Example verification:
Suppose we had an additional 0.50 g of \(Cl_2\) in the reaction. Would all of the K be consumed, or would there still be excess K?
Moles of additional \(Cl_2\) = mass of \(Cl_2\) / molar mass of \(Cl_2\)
Moles of additional \(Cl_2\) = 0.50 g / 70.90 g/mol
Moles of additional \(Cl_2\) = 0.0070 mol
The theoretical yield of KCl that can be formed from the additional \(Cl_2\) is:
0.0070 mol \(Cl_2\) x (2 mol KCl / 1 mol \(Cl_2\)) x (74.55 g KCl / 1 mol KCl) = 1.04 g KCl
Therefore, the total amount of KCl that can be formed from all of the \(Cl_2\) is:
5.95 g + 1.04 g = 6.99 g
The amount of K that would be needed to completely consume all of the \(Cl_2\).
Learn more about Solid Potassium at
brainly.com/question/27549056
#SPJ1
Which compound is a strong electrolyte?
Please choose the correct answer from the following choices, and then select the submit answer button.
Answer choices
Be(OH)2
BeS
Be3(PO4)2
Be(NO3)2
Answers
Be(OH)₂ compound is a strong electrolyte.
What is strong electrolyte?A solid or liquid that totally or almost completely ionises or dissociates in a solution is considered a strong electrolyte. In the solution, these ions function well as electrical conductors. An early definition of a "strong electrolyte" was a substance that is an effective conductor of electricity when dissolved in water. Strong electrolytes are those that totally dissociate or ionise when placed in aqueous solution. These electrolytes have a high electrical conductivity and a greater ionisation extension. NaCl, HCl, NaOH, etc. are a few examples.
Correct option: Be(OH)₂
To know more about strong electrolyte refer to:
https://brainly.com/question/1581652
#SPJ1
Calculate the number of CO2
molecules ( NCO2
) in 0.0734 mol
of CO2
Answers
Answer:
4.42 x 10^22 molecules
Explanation:
1 mole of CO2 has 6.022 x 10^23 molecules
=> 0.0734 x 6.022 x 10^23 = 4.420148 x 10^22 or 4.42 x 10^22
Select the correct answer.
What is a nonpolar covalent bond?
A.
a bond between two nonmetal atoms
B.
a bond in which electrons are shared unequally
C.
a bond with ΔEN greater than 0.5
D.
a bond between two atoms that have equal electronegativities
Answers
D. a bond between two atoms that have equal electronegativities
Explanation:Covalent bonds involve 2 atoms sharing electrons.
Covalent Bonds
There are 3 types of bonds: metallic, ionic, and covalent. Metallic bonds occur between 2 metals that exist in a "sea of electrons." Ionic bonds have high electronegativity differences and occur between a metal and a nonmetal. Finally, as stated above, covalent bonds occur when 2 atoms share their electrons. Covalent bonds usually occur between two nonmetals. However, there are 2 types of covalent bonds: polar and nonpolar.
Nonpolar Bonds
Both polar and nonpolar bonds involve the sharing of electrons; however, polar bonds share electrons unequally. This is caused by an electronegativity difference greater than 0.5. When two atoms have equal electronegativities, they share the electrons equally. This creates a nonpolar bond.
1.(a) Describe a condensation (dehydration synthesis) reaction. What type of organic molecule (that we discussed in class) forms as a result of such reactions?(b) Describe an addition reaction. Do saturated or unsaturated organic molecules participate in addition reactions? Explain your reasoning.
Answers
(a) Condensation reaction is a reaction whereby two molecules combine to form a single molecule. During this reaction, water is removed. For example, two amino acids combine by a covalent bond, water molecule is then removed as a second product. Esterification is a type of condensation reaction, whereby an ester is formed from an alcohol and a carboxylic acid. Another example of condensation reaction is saponification which describes the alkaline hydrolysis reaction of an ester.
(b) Addition reaction is a reaction whereby a double bond is broken by additing an element. Unsaturated organic molecules participate in addition reactions, this is because unsaturated organic molecules are those that have double bonds.
For example addition of hydrogen: Addition of hydrogen to a carbon-carbon double bond is called hydrogenation, which results in the removal of the double bond.
An avocado has a mass of 215 g and a density of 0.86
g/mL. What is the volume of the avocado?
Answers
.86 = 215/volume
.86(volume) = 215
volume = 250
The volume of avocado here is 250 ml as per the given data i.e., mass of 215 g and a density of 0.86 g/mL.
What is density?The density of an artifact is delineated as its mass divided by the volume. Density is commonly expressed in grams per cubic centimeter.
The grams are a unit of mass and indeed cubic centimeters are a unit of volume. A box with more particles will be denser than a box with fewer particles.
The density of a solid, liquid, or gas illustrates how closely packed the particles can be. The amount of mass per unit volume is defined as density.
Density is defined as d = M/V, where d is density, M is mass, and V is volume. Density is commonly measured in grams per cubic centimetre.
We know that,
Density = mass/volume.
Volume = mass/density.
Here, it is given that
Density = 0.86 g/mL.
Mass = 215 g.
Volume = 215/0.86 mL.
Volume = 250 mL.
Thus, the answer is 250 mL.
For more details regarding density, visit:
https://brainly.com/question/15164682
#SPJ5
how many moles of atoms of each element in one mole (NH4)2CO3
Answers
Answer:
Chemists generally use the mole as the unit for the number of atoms or molecules of a material. One mole (abbreviated mol) is equal to 6.022×1023 molecular entities (Avogadro's number), and each element has a different molar mass depending on the weight of 6.022×1023 of its atoms (1 mole).\
Have a great day!!!!
Explanation:
1 mole of (NH₄)₂CO₃ has:
2 moles of N atoms.8 moles of H atoms1 mole of C atoms.3 moles of O atoms.Let's consider the chemical formula of ammonium carbonate: (NH₄)₂CO₃.
The subscripts inform us of the number of moles of atoms in 1 mole of molecules.
Nitrogen has 2 subscripts: "1" (which is not written) and "2" (outside the parenthesis). The global subscript for nitrogen is obtained by multiplying both subscripts.
\(N: 1 \times 2 = 2\)
Hydrogen has 2 subscripts: "4" and "2" (outside the parenthesis). The global subscript for nitrogen is obtained by multiplying both subscripts.
\(H: 4 \times 2 = 8\)
The subscript for carbon is "1".
The subscript for oxygen is "3".
1 mole of (NH₄)₂CO₃ has:
2 moles of N atoms.8 moles of H atoms1 mole of C atoms.3 moles of O atoms.Learn more: https://brainly.com/question/375587
If NaCl has a mass of 3.2g, what is the volume of chlorine gas at STP?
Answers
Hey there!
Molar mass NaCl = 58.44 g/mol
58.44 g ----------------- 22.4 ( at STP )
3.2 g -------------------- Volume ??
Volume = ( 3.2 x 22.4 ) / 58.44
Volume = 71.68 / 58.44
Volume = 1.226 L
Hope this helps!
Answer:
V = 0.56 L
Explanation:
Given data:
Mass of NaCl = 3.2 g
Volume of chlorine gas = ?
Pressure and temperature = standard
Solution:
Chemical equation:
2NaCl → 2Na + Cl₂
Number of moles of NaCl:
Number of moles = mass/molar mass
Number of moles = 3.2 g/ 58.44 g/mol
Number of moles = 0.05 mol
Now we will compare the moles of NaCl with chlorine;
NaCl : Cl₂
2 : 1
0.05 : 1/2×0.05 = 0.025 mol
Volume of chlorine:
PV = nRT
V = nRT/P
V = 0.025 mol × 0.0821 atm.L/mol.K × 273 K / 1 atm
V = 0.56 L