a 5.282 g sample of a solid, weak, monoprotic acid is used to make a 100.0 ml solution. 30.00 ml of the resulting acid solution is then titrated with 0.09459 m naoh. the ph after the addition of 24.00 ml of the base is 5.01, and the endpoint is reached after the addition of 45.78 ml of the base. please see titration to determine molecular weight for assistance. (a) how many moles of acid were present in the 30.00 ml sample?
Answers
The number of moles of acid present in the 100.0 mL solution is 0.001444 mol.
The balanced chemical equation for the reaction is:
acid + NaOH → salt + water
Since the acid is monoprotic, we can assume that one mole of acid reacts with one mole of NaOH. Therefore, the number of moles of acid present in the 30.00 mL sample is equal to the number of moles of NaOH added at the endpoint of the titration. We can calculate the number of moles of NaOH using the formula:
moles NaOH = concentration of NaOH × volume of NaOH
moles NaOH = 0.09459 mol/L × 45.78 mL / 1000 mL/mL
moles NaOH = 0.004332 mol
Since one mole of acid reacts with one mole of NaOH, the number of moles of acid present in the 30.00 mL sample is also 0.004332 mol.
Now we need to find the number of moles of acid present in the original 100.0 mL solution. We can use the formula:
moles acid = mass of acid / molecular weight of acid
The molecular weight of the acid can be determined by using the titration data to calculate the molarity of the acid solution. From the titration data, we know that:
moles NaOH at endpoint = moles acid in 30.00 mL
moles NaOH at endpoint = 0.004332 mol
volume of acid in 100.0 mL = 30.00 mL / 100.0 mL × 1000 mL/L = 300.0 mL/L
molarity of acid = moles acid/volume of acid in L
molarity of acid = 0.004332 mol / 0.3000 L
molarity of acid = 0.01444 mol/L
Now we can use the molarity and volume of the acid solution to calculate the number of moles of acid in the 100.0 mL solution:
moles acid = molarity of acid × volume of acid in L
moles acid = 0.01444 mol/L × 0.1000 L
moles acid = 0.001444 mol
Therefore, the number of moles of acid present in the 100.0 mL solution is 0.001444 mol.
learn more about monoprotic acid here
https://brainly.com/question/28556909
#SPJ1
Related Questions
which of the following molecules/ions will exhibit delocalized pi (π) bonding? group of answer choices of2 o2 cocl2 co2 co32-
Answers
Out of the given molecules and ions, CO32- will exhibit delocalized pi (π) bonding.
Delocalized π bonding occurs when the π electrons in a molecule are not confined to a single bond between two atoms, but instead spread out over multiple atoms or a larger area of the molecule. In CO32-, the central carbon atom has a double bond with each of the two oxygen atoms and a single bond with the third oxygen atom.
The π electrons in these double bonds are shared between all three oxygen atoms, creating a delocalized π bond. This delocalization allows for greater stability and makes the molecule less reactive. The other molecules and ions listed do not have delocalized π bonding because their π electrons are confined to a single bond between two atoms.
Learn more about molecules at https://brainly.com/question/17930825
#SPJ11
Is CuS an ionic, non polar or polar covalent bond and why
Answers
Answer:
Ionic
Explanation:
Answer:
Ionic Bond
Explanation:
CuS has an ionic bond between it as it it formed by the combination of two ions Cu⁺² and S⁻².
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Having a control group is important when doing an investigation why?
Answers
Answer:to help understand the stuff more
Explanation:
Answer :
Dependent variable depends on the independent variable. ... In a control group in an experimental investigation the independent variable is omitted. Thus the goal is to test if the dependent variable shows any change in the absence of the independent variable.
Which is the smallest volume?
A
2.3 liters
B
0.4 mL
С
980 cm
D
1050 mL
heat
Answers
34.8 g of Na₂O are used to form a solution with a volume of 0.50 L. What is the molarity?
Answers
Answer:
Molarity = 1.12 mol/L
Explanation:
To make an aqueous solution of Na₂O, the concentration will be calculated by: concentration (c) (or molarity) = number of moles present (n) ÷ volume needed (V) (in litres)
since we don't have moles, we can calculate moles by:
number of moles (n) = mass present (m) (in grams) ÷ molar mass (M) (in grams per mole), which we can find using a standard IUPAC Periodic Table
∴ n(Na₂O) = m/M = 34.8/(22.99×2+16.00) = 0.56147 mol
Now we have the number of moles present, we can calculate concentration:
∴ c(Na₂O) = n/V = 0.56147/0.50L = 1.12 mol/L
Explain the concept law of diminishing marginal rate of substitution. What is/are the reason/s why the law of diminishing marginal rate of substitution suggest/s that isoquant must be bent toward the origin?
Answers
The law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
In other words, as the quantity of one good increases, the individual is willing to sacrifice fewer units of the other good to obtain an additional unit of the first good. This reflects a diminishing rate of substitution between the two goods.
The reason why the law of diminishing marginal rate of substitution suggests that isoquants must be bent toward the origin is rooted in the concept of diminishing marginal utility. As more units of a particular input (e.g., labor or capital) are added while holding other inputs constant, the additional output gained from each additional unit of the input will decrease. This diminishing marginal productivity leads to a decreasing MRS.
When isoquants (which represent different combinations of inputs that produce the same level of output) are bent toward the origin, it reflects the fact that as more of one input is used, the amount of the other input that needs to be substituted decreases. This bending signifies the diminishing MRS and captures the idea that a larger quantity of one input can be substituted for a smaller quantity of the other input to maintain the same level of output.
Overall, the law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
To know more about marginal rate of substitution, click here, https://brainly.com/question/30763866
#SPJ11
Calculate the pH during the titration of 20.00 mL of 0.1000 M ammonia, with 0.2000 M HCl(aq) after 6.07 mL of the acid have been added.
Answers
When an acid is added to an alkaline solution such as ammonia, the pH of the solution decreases. This is because hydrogen ions, which are acidic, are added to the solution. The pH of the solution is calculated using the formula pH = -log[H+].Given information:Initial volume of ammonia solution = 20.00 mLInitial concentration of ammonia solution = 0.1000 MVolume of HCl added = 6.07 mL Concentration of HCl solution = 0.2000 M1.
Write the balanced chemical equation for the reaction that takes place during the titration of ammonia with hydrochloric acid. NH3 + HCl → NH4Cl2. Calculate the number of moles of ammonia present initially. n = C x V where ,n = number of mole C = concentrationV = volume Substituting the values, we getn(NH3) = 0.1 mol/L x (20.00/1000) L= 0.002 mol3. Calculate the number of moles of HCl added. n(HCl) = C x Vwhere,n = number of moles C = concentrationV = volumeSubstituting the values, we getn(HCl) = 0.2 mol/L x (6.07/1000) L= 0.001214 mol4. Calculate the number of moles of ammonia remaining. At this stage, all the hydrochloric acid added will react with ammonia to form ammonium chloride. The amount of hydrochloric acid added is less than the amount required to completely react with ammonia. Hence, some ammonia will remain unreacted.n(NH3) remaining = n(initial) - n(HCl added)= 0.002 - 0.001214= 0.000786 mol5. Calculate the volume of hydrochloric acid added when all the ammonia has reacted. From the balanced equation, the stoichiometry of ammonia and hydrochloric acid is 1:1. Hence, the number of moles of ammonia that has reacted is equal to the number of moles of hydrochloric acid added. n(HCl) reacted = 0.000786 moln(HCl) added at equivalence point = n(HCl) reacted + n(HCl) initially= 0.000786 + 0.001214= 0.002 molV(HCl) added at equivalence point = n(HCl) / CV(HCl) = 0.002 mol / 0.2 mol/L= 0.01 L or 10 mL6. Calculate the pH after 6.07 mL of hydrochloric acid has been added. At this stage, the number of moles of hydrochloric acid added is less than the number of moles required to reach equivalence point. Hence, the hydrochloric acid added is limiting, and the pH is calculated using the Henderson-Hasselbalch equation.pH = pKa + log([base]/[acid])At this stage, ammonia is the base and ammonium chloride is the acid.pKa for NH4+/NH3 = 9.25[base] = n(NH3) remaining = 0.000786 mol[acid] = n(HCl added) = 0.001214 molSubstituting the values, we getpH = 9.25 + log(0.000786/0.001214)= 9.25 - 0.121= 9.13Therefore, the pH of the solution after 6.07 mL of hydrochloric acid has been added is 9.13.For such more question on mole
https://brainly.com/question/29367909
#SPJ11
a beaker containing 50.0 ml of 0.50 m naoh is titrated using a burette containing a solution of 0.50 m hcl. report all answers to two decimal places (e.g. 1.00) prompt 1what is the resulting ph of the solution if only 25.0 ml of titrant is added? answer for prompt 1 what is the resulting ph of the solution if only 25.0 ml of titrant is added? prompt 2what is the resulting ph of the solution if 50.0 ml of titrant is added? answer for prompt 2 what is the resulting ph of the solution if 50.0 ml of titrant is added? prompt 3what is the resulting ph of the solution if 75.0 ml of titrant is added?
Answers
The pH of the solution after 28.0 ml of NaOH have been added to the acid exists 0.85.
How to estimate the ph of the solution?The balanced reaction between base and acid exists;
NaOH + HCl → NaCl + H₂O
NaOH exists a strong base and HCl exists a strong acid therefore complete dissociation. Stoichiometry of acid to base exists 1: 1.
The number of moles of base added - 0.5 M /1000 mL/L × 28.0 mL = 0.014 mol
the number of acid moles present - 0.5 M /1000 mL/L × 50.0 mL = 0.025 mol.
acid reacts with base 1: 1 ratio
Therefore excess amount of acid present - 0.025 - 0.014 = 0.011 mol
Total volume = 50.0 + 28.0 = 78.0 mL
[H⁺] = 0.011 mol/0.078 L = 0.14 M
Therefore, pH = -log [H⁺]
substitute the values in the above equation, we get
pH = -log(0.14)
simplifying the above equation, we get
pH = 0.85.
Therefore, the pH of the solution after 28.0 ml of NaOH have been added to the acid exists 0.85.
To learn more about pH values refer to:
https://brainly.com/question/172153
#SPJ4
For each set below rank the bonds according to strength.A) Cl-Cl, I-I, Br-BrB) Si-F, Si-I, Si-Cl
Answers
For the first set, we can rank the bonds according to strength based on their bond dissociation energies (BDEs).Thus, the ranking from strongest to weakest is I-I, Br-Br, Cl-Cl.
For the second set, we can use a similar approach.Chlorine is in between. Thus, the ranking from strongest to weakest is Si-F, Si-Cl, Si-I.
A) In the first set, we have the halogens Cl-Cl, I-I, and Br-Br. The bond strength depends on the size of the atoms and the overlapping of their electron clouds. As we move down the periodic table, atomic size increases, resulting in weaker bond strength due to less overlap between electron clouds. So, the ranking of bond strength for set A would be as follows:
1. Cl-Cl (strongest)
2. Br-Br
3. I-I (weakest)
B) In the second set, we have Si-F, Si-I, and Si-Cl. In this case, bond strength is determined by the difference in electronegativity between silicon and the other atoms. The greater the electronegativity difference, the stronger the bond. The electronegativity values for silicon, fluorine, iodine, and chlorine are approximately 1.8, 3.98, 2.66, and 3.16, respectively. Based on these values, the ranking of bond strength for set B would be:
1. Si-F (strongest)
2. Si-Cl
3. Si-I (weakest)
Learn more about bond dissociation energies here:-
https://brainly.com/question/30762022
#SPJ11
Two samples of sodium chloride were decomposed into their constituent elements. One sample produced 2.84 g of sodium and 4.37 g of chlorine. Which of the following could be the results of the decomposition of the other sample, being consistent with the law of constant composition (also called the law of definite proportions or law of definite composition)?
a) 4.17 g of sodium and 3.75 g of chlorine
b) 4.17 g of sodium and 6.42 g of chlorine
c) 4.17 g of sodium and 1.05 g of chlorine
d) 4.17 g of sodium and 12.1 g of chlorine
Answers
Answer:
The correct answer is b) 4.17 g of sodium and 6.42 g of chlorine
Explanation:
According to the law of definite proportions a chemical compound is composed always by the same elements in the same proportions by mass. In this case, the proportion of the elements by mass will be 4.37 g of chlorine (Cl) per 2.84 g of sodium (Na):
4.37 Cl/2.84 g Na= 1.54
We can calculate the proportions of the results in order to see which is the correct:
a) 3.75 g Cl/4.17 g Na = 0.899
b) 6.42 g Cl/4.17 g Na = 1.539 ⇒ ≅1.54
c) 1.05 g Cl/4.17 g Na = 3.971
d) 12.1 g Cl/4.17 g Na = 2.901
The option in which the proportion Cl/Na is equal to 1.54 is option b
Please help what is the answer
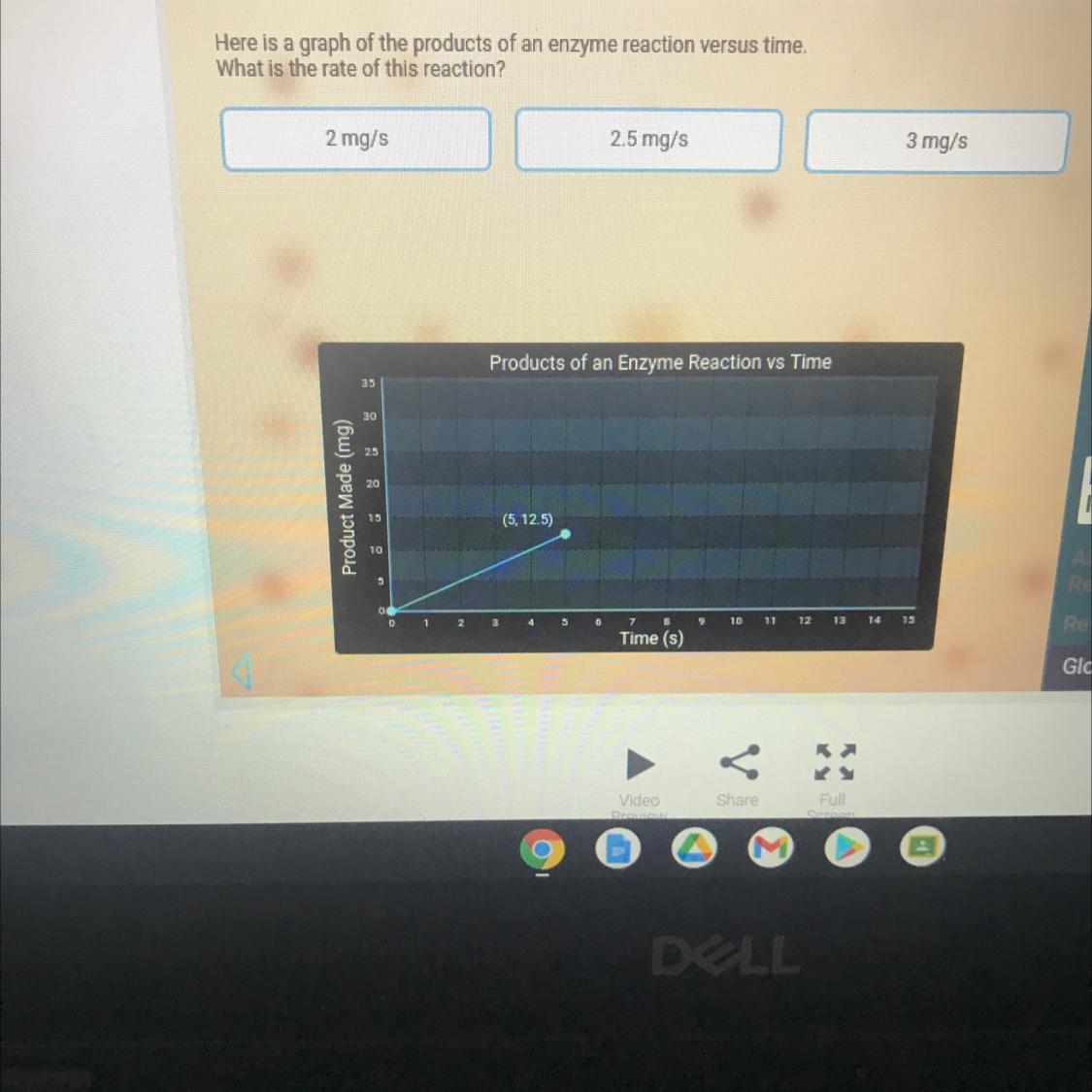
Answers
Answer:
the secend on 2.5mg/s
Explanation:
Why would it be more difficult to inject the nucleus of uranium with a proton?
Answers
Answer:
Because there are already 92 protons in the
uranium nucleus, combining their repulsive power to reject the one new one. However, if the energy of the incoming proton is sufficiently great, then at extremely close distance, the uranium nucleus will attract the proton and draw it into the nucleus. This is called the strong force.
16 S 33
Explanation:
I hope it helped
150.0 grams of zinc nitrate are how many moles?
Answers
150g•1moles/189.36g= 0.792moles
Which statement about the purpose of the field of science is false

Answers
Science works to establish a collection of unchanging truths is false about the purpose of the field of science.
What does the word "science" mean?
Science is the methodical, empirically-based pursuit and application of knowledge and understanding of the natural and social worlds. Included in scientific methodology are the following: unbiased observation metric and information (possibly although not necessarily using mathematics as a tool) Evidence.
The fields of science known as mathematics, physics, chemistry, and biology are referred to as the basic sciences. They are referred to as basic sciences because they offer a fundamental comprehension of natural occurrences and the procedures that change natural resources.
To learn more about science use link below:
https://brainly.com/question/18186842
#SPJ1
If sodium bicarbonate is added to an aqueous solution of ethanol, will carbon dioxide gas evolve?
Answers
No, carbon dioxide gas will not evolve when sodium bicarbonate is added to an aqueous solution of ethanol.
Sodium bicarbonate (NaHCO3), commonly known as baking soda, is a weak base. When it reacts with an acid, such as acetic acid or citric acid, carbon dioxide gas (CO2) is produced as a byproduct. However, in the given scenario where sodium bicarbonate is added to an aqueous solution of ethanol, which is not acidic, there is no acid present to react with the sodium bicarbonate. As a result, there is no chemical reaction that would release carbon dioxide gas. Therefore, the addition of sodium bicarbonate to an aqueous solution of ethanol would not lead to the evolution of carbon dioxide gas.
Learn more about sodium bicarbonate here:
https://brainly.com/question/28721969
#SPJ11
what is the kinetic energy of an object with velocity of 7m /s and a 14kg mass?
Answers
Answer:
343 JExplanation:
The kinetic energy of an object can be found by using the formula
\(k = \frac{1}{2} m {v}^{2} \\ \)
m is the mass
v is the velocity
We have
\(k = \frac{1}{2} \times 14 \times {7}^{2} \\ = 7 \times49 \\ = 343 \: \: \: \: \: \)
We have the final answer as
343 JHope this helps you
Can someone help me please!

Answers
Which family group on the peirodic table has the most elements
a. metals
b. nonmetals
c. trainsiton elements
d. metalloids
Answers
Answer:
C. Transition metals
Explanation:
Out of the answers transition metals have the most elements on the periodic table.
A bicycle tire has a volume of 1. 0 L at 22°C. If the temperature of the tire is increased to 52°C, what is the resulting volume of the tire? Type in your answer using the correct number of significant figures. Use the formula for Charles' Law: V1 T1 = V2 T2 L.
Answers
The resulting volume of the tire would be 1.1 L
What is Charles's law?According to this law, the volume of a given gas is directly proportional to its temperature, provided that the pressure remains constant.
The law can be presented mathematically as;
\(\dfrac{V_1}{T_1} =\dfrac{V_2}{T_2}\)
In this case, V1 = 1.0 L, T1 = 22 °C (295 K), T2 = 52 °C (325 K).
\(V_2=\dfrac{V_1T_2}{T_1}\)V
\(V_2=\dfrac{1\times 325}{295}=1.1\ L\)
Hence the final volume of the tire will be 1.1 L
More on Charles's law can be found here:
brainly.com/question/3491421
Calculate the number of copper atoms in 2.45 mole of copper
Answers
Answer:
1.48×10²⁴ Cu atoms
Explanation:
For this question you need to use Avogadro's number 6.022×10²³atoms.
2.45 moles of Cu ×\(\frac{6.022x10x^{23} atoms }{1 mole}\)= 1.47539×10²⁴ atoms.
The moles cancel out so you are left with atoms.
Since there are 3 significant figures in the question there should be 3 significant figures in your answer, which is 1.48×10²⁴ Cu atoms.
to the best of your knowledge, classify each of the following as an element, compound, or mixture. explain how your everyday experiences influenced your response. a. silver coin b. air c. coffee d. soil
Answers
a. Silver coin - Element (Silver is a pure element and is not chemically combined with any other element in a silver coin)
b. Air - Mixture (Air is a mixture of gases, primarily nitrogen and oxygen, with trace amounts of other gases and particles)
c. Coffee - Mixture (Coffee is a mixture of various compounds such as water, caffeine, and organic compounds that give it its flavour and aroma)
d. Soil - Mixture (Soil is a mixture of various substances such as minerals, organic matter, water, and air)
My everyday experiences influenced my response because I have come across these examples in my daily life and have been taught about them in science classes. For example, I know that air is composed of different gases and particles, and that soil is made up of a mixture of substances, including minerals and organic matter. Similarly, I know that a silver coin is made of pure silver and that coffee is made of various compounds.
To learn more about Compounds click here:
https://brainly.com/question/14658388
#SPJ4
What are the general trends in first ionization energy across a period (LR) within the
periodic table? From bottom to top within a group?
Answers
If you want to make 8.00 moles of AlF₃ how many moles of F₂ will you need, using the following balanced chemical equation? 2 Al + 3 F₂ → 2 AlF₃
Answers
Answer:
You will need 12.0 moles of F₂
Explanation:
Based on the chemical equation, 2 moles of AlF₃ are produced when 3 moles of F₂ are added in an excess of Al.
Using this expression: 2mol AlF₃ = 3mol F₂ we can find the moles of F₂ as follows:
8.00 mol AlF₃ * (3mol F₂ / 2mol AlF₃) =
You will need 12.0 moles of F₂the process of _____ determines a substance’s physical or chemical identity with as near absolute certainty as existing analytical techniques will permit.
Answers
The process of spectroscopy determines a substance's physical or chemical identity with as near absolute certainty as existing analytical techniques will permit.
Spectroscopy is the study of the interaction of electromagnetic radiation with matter and is a crucial tool in chemical analysis. By studying the spectrum of a substance (the way it absorbs, emits, or reflects light), spectroscopy can provide information about the composition, structure, and properties of the substance, thereby allowing for its identification with high accuracy.
Spectroscopy is a broad and interdisciplinary field that encompasses many different techniques for studying the interaction of electromagnetic radiation with matter. It plays a crucial role in various areas of science, including physics, chemistry, biology, and materials science. Spectroscopy provides information about the composition, structure, and properties of substances by analyzing the way they interact with light.
Learn more about spectrum here:
https://brainly.com/question/6836691
#SPJ4
Attempt 1 of 1
Which of the following is most likely to have a crystalline structure?
wood
rubber
glass
quartz
Answers
Answer: Quartz
Explanation: I looked it up ;)
based on the size and polarity of the molecules, for which pair of compounds below do both compounds have a smell?
Answers
Based on the size and polarity of the molecules, for NH\(_3\) and C\(_{10}\)H\(_{18}\)O pair of compounds have a smell.
Any substance made up of similar molecules with atoms from two or more different chemical elements is referred to as a chemical compound. Atoms comprising more than 100 distinct chemical compounds make up all of the matter throughout the cosmos.
It can be found both alone and in combination as chemical compounds. A sample of a pure element contains only the atoms that are distinctive of that element, because each element's atoms are distinct. Based on the size and polarity of the molecules, for NH\(_3\) and C\(_{10}\)H\(_{18}\)O pair of compounds have a smell.
To know more about chemical compound, here:
https://brainly.com/question/12166462
#SPJ1
Calculate the volume (in L) occupied by 3.40 g of NH3 at STP. (STP: 0 degree Celsius and 1 atmosphere of pressure).
Answers
Answer:
Calculate the volume (in L) occupied by 3.40 g of NH3 at STP. (STP: 0 degree Celsius and 1 atmosphere of pressure).
Explanation:
To get the volume of ammonia gas at STP, calculate the number of moles of \(NH_3\) in the given amount.
Number of moles of \(NH_3\) gas is:
\(number of moles of NH_3 gas&=\frac{given mass of the gas}{its molecular mass} \\ &=\frac{3.40g}{17.0g/mol} \\&=0.2 mol.\)
Since,
1 mol of any gas at STP occupies ------- 22.4 L of volume.
then,
0.2 mol of \(NH_3\) occupies how much volume?
\(=>0.2 mol x \frac{22.4 L}{ 1.0 mol} \\=4.48 L\)
Hence, the volume occupied by 3.40 g of ammonia at STP is --- 4.48 L.
When compared to sulfuric acid, how strong are carboxylic acids? stronger just as strong weaker not acidic at all
Answers
We can see here that when compared to sulfuric acid, carboxylic acids are weaker acids.
What is sulfuric acid?Sulfuric acid is a strong, highly corrosive acid with the chemical formula \(H_{2} SO_{4}\). It is one of the most important and widely used industrial chemicals. Sulfuric acid is known for its strong acidic properties and is often referred to as the "king of chemicals" due to its widespread applications in various industries.
Sulfuric acid is a dense, oily liquid that is colorless when pure. It is soluble in water, and when dissolved, it releases hydrogen ions (H+) to make the solution highly acidic.
Learn more about sulfuric acid on https://brainly.com/question/10220770
#SPJ4
An atom of which element has the greatest attraction for the electrons in a bond with a hydrogen atom
Answers
Answer: Fluorine
Explanation: There are many variables that can affect the strenght of attraction of the electrons in a chemical bond.
The most significant of them is the electronegativity: a meassure of the affinity an element has with the electrons . The higher the electronegativity, the stronger the attraction with electrons.
Brainliest pls Have a nice day