Answers
Answer:
2.... is the answer..
Hope it helps!!!
Answer:
2 is the answer
Explanation:
Am correct
Related Questions
7) How many molecules of CO2 are in 2.5 L at STP?
Answers
By using the ideal gas law and Avogadro's number, we find that there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
To determine the number of molecules of CO2 in 2.5 L at STP (Standard Temperature and Pressure), we can use the ideal gas law and Avogadro's number.
Avogadro's number (N_A) is a fundamental constant representing the number of particles (atoms, molecules, ions) in one mole of substance. Its value is approximately 6.022 × 10^23 particles/mol.
STP conditions are defined as a temperature of 273.15 K (0 °C) and a pressure of 1 atmosphere (1 atm).
First, we need to convert the volume from liters to moles of CO2. To do this, we use the ideal gas law equation:
PV = nRT,
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we have STP conditions, we can substitute the values:
(1 atm) × (2.5 L) = n × (0.0821 L·atm/(mol·K)) × (273.15 K).
Simplifying the equation:
2.5 = n × 22.4149.
Solving for n (the number of moles):
n = 2.5 / 22.4149 ≈ 0.1116 moles.
Next, we can calculate the number of molecules using Avogadro's number:
Number of molecules = n × N_A.
Number of molecules = 0.1116 moles × (6.022 × 10^23 particles/mol).
Number of molecules ≈ 6.72 × 10^22 molecules.
Therefore, there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
For more such questions on ideal gas law visit:
https://brainly.com/question/27870704
#SPJ8
Helium is located in group 8A but does not have eight valence electrons. Why is it located in 8A, not 2A?
Answers
Answer:
its outermost shell is completely full making it extremely stable.
Explanation:
It only has two electrons in its outer shell so its valence electron configuration is 1s2. Even though it only has two electrons, it is grouped with elements that have eight valence electrons. Helium is still happy because its outermost shell is completely full making it extremely stable.
Samples of different materials, A and B, have the same mass, but the sample
of A is higher in density. Which statement could explain why this is so?
A. The sample of material A has greater volume than the sample of
material B.
B. The particles that make up material B are more closely packed
together than the particles that make up material A.
C. The particles that make up material A have more mass than the
particles that make up material B.
D. The particles that make up material B have more mass than the
particles that make up material A.
SUBMI

Answers
C. The particles that make up material A have more mass than the particles that make up material B.
The correct option is (c) The particles that make up material A have more mass than the particles that make up material B.
A substance containing atoms that are smaller, more massive, and close together will have a higher density. A substance will have a lower density if its larger, lighter atoms are spaced farther apart.The distances between the particles in each state of matter determine how dense solids, liquids, and gases are.Will the density of a material always be the same?Density is an intensive property. This means that regardless of the object's shape, size, or quantity, the density of that substance will always be the same. Even if you cut the object into a million pieces, they would still each have the same density. It is because density in an intensive property of matter.Learn more about Density of a material brainly.com/question/1733081
#SPJ2
Animal fats and vegetable oils are triacylglycerols, or triesters, formed from the reaction
of glycerol (1, 2, 3-propanetriol) with three long-chain fatty acids. One of the methods
used to characterize a fat or an oil is a determination of its saponification number. When
treated with boiling aqueous KOH, an ester is saponified into the parent alcohol and fatty
acids (as carboxylate ions). The saponification number is the number of milligrams of
KOH required to saponify 1.000 g of the fat or oil. In a typical analysis, a 2.085-g sample
of butter is added to 25.00 mL of 0.5131 M KOH. After saponification is complete, the
excess KOH is back titrated with 10.26 mL of 0.5000 M HCl. What is the saponification
number for this sample of butter?
Answers
Saponification number = (V × M × F × 56.1) / W
Where:
V = volume of HCl used in the back titration
M = molarity of HCl
F = factor of KOH (which is 1 for pure KOH)
W = weight of the butter sample used in grams
First, we need to calculate the amount of KOH used in the saponification reaction:
0.5131 M KOH = 0.5131 moles KOH / liter
25.00 mL KOH = 0.02500 L KOH
moles KOH used = 0.5131 moles/L × 0.02500 L = 0.0128 moles KOH
Since the saponification reaction is a 1:1 reaction between KOH and the triacylglycerol in the butter sample, the amount of butter used is also 0.0128 moles.
Next, we need to calculate the amount of HCl that reacted with the excess KOH:
0.5000 M HCl = 0.5000 moles HCl / liter
10.26 mL HCl = 0.01026 L HCl
moles HCl used = 0.5000 moles/L × 0.01026 L = 0.00513 moles HCl
Since the reaction between HCl and KOH is also a 1:1 reaction, the moles of KOH that were not used in the saponification reaction is equal to the moles of HCl used in the back titration:
moles KOH not used = moles HCl used = 0.00513 moles HCl
To find the saponification number,
Saponification number = (V × M × F × 56.1) / W
Saponification number = (0.01026 L × 0.5000 moles/L × 1 × 56.1) / 2.085 g
Saponification number = 6.50
Therefore, the saponification number for this sample of butter is 6.50.
To know more about Saponification:
https://brainly.com/question/2263502
#SPJ1
3- Write the chemical formula of Alanine and find to which food category it belongs.
Answers
Answer:
C3H7NO2
Explanation:
The chemical formula of Alanine is C3H7NO2 and it belongs to almost all food category because it is present in one food of each food category such as dairy products, meat, nuts, soy, and whole grains etc. Alanine is a nonessential amino acid means that it does not directly obtained from the diet but it can be produced by the body using raw materials present in the food.
A car travels at a speed of 54 km/hr. How many meters will it travel in 1 second?
Answers
Answer: 15 METERS IN A SECOND
Explanation: 54km = 54000m 54000m / 60 = 900 900/60 = 15
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
please help quick will give brainliest
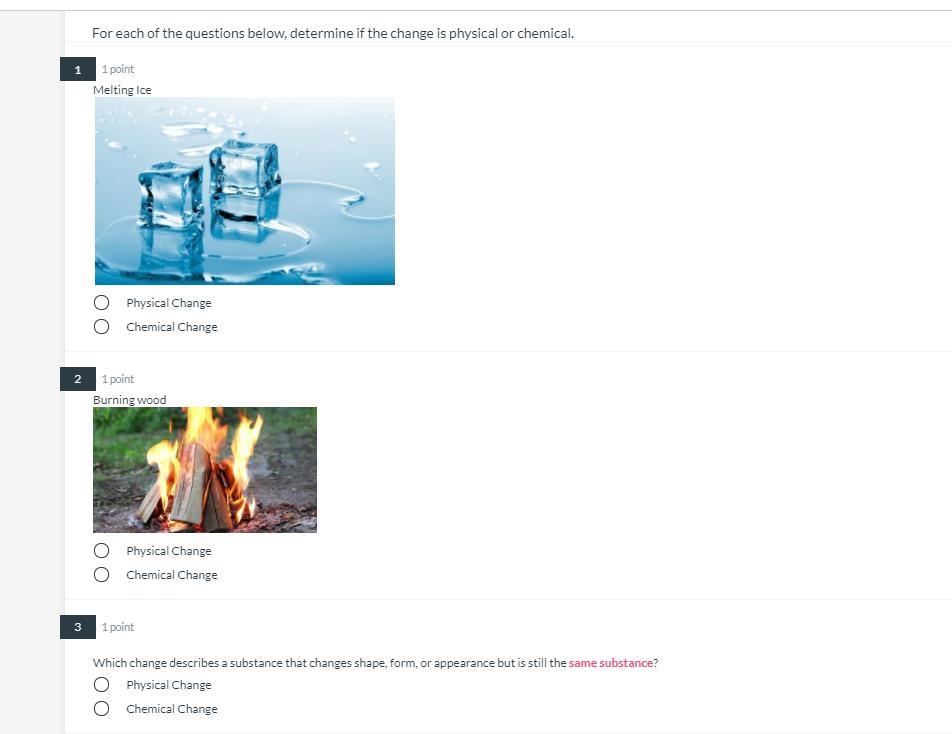
Answers
Answer:
I think it's Physical, Chemical, then Physical
Explanation:
I hope I'm right, those answers sound about right to me
PLEASE HELPPP!!!!!!!!

Answers
Explanation:
Label A: oceanic - oceanic
Label B: oceanic - continental
Label C: continental - continental
Answer:
Oceanic-continental convergence---- letter B.
Oceanic-oceanic convergence ---- letter A.
Continental-continental convergence ---- letter C
plz mark brainliest
Which statement BEST explains why the Moon is visible from the Earth?
1) The Moon reflects light from the Sun.
2) The Moon is always lit up by the Sun for us to see.
3) The Moon reflects light from the Earth.
4) The Moon produces its own light through radiation.
Answers
Answer: The answer is the ''The moon is always lit up by the sun for us to see.''
Explanation: The moon is directly illuminated by the sun.
Consider the following reaction profile:
M
potential energy
reaction coordinate
How many steps are in the reaction? [Select]
Which step is the rate-limiting step? [Select]
How many transition states exist in this reaction? [Select]
Is AE for this reaction positive or negative? [Select]

Answers
From the question;
1) There are two steps in the reaction
2) The first step is the rate limiting step
3) There are two transition states in the reaction
4) ΔE for the reaction is positive
What is the reaction profile?An energy profile, reaction energy diagram, or reaction profile is a graphical depiction of the energy changes that take place during a chemical process. The energy of the reactants, the energy of the products, and the energy changes that occur when the reactants are transformed into products are all illustrated.
A horizontal axis that shows the reaction's progress and a vertical axis that reflects the energy make up the reaction profile in most cases. The system's potential energy is used to calculate the energy.
Learn more about reaction:https://brainly.com/question/30464598
#SPJ1
(b) Two compounds, A and B, have the molecular formula C₂H6O. On treatment with Na metal, compound A releases H2 gas and compound B does not.
Can you give a reason to help to explain the observation better?
Answers
The observation that compound A releases H2 gas while compound B does not when treated with Na metal can be explained by considering the structural differences between the two compounds and their ability to undergo specific reactions.
Compound A and compound B both have the molecular formula C₂H₆O, which indicates that they both contain two carbon atoms, six hydrogen atoms, and one oxygen atom. However, the difference lies in the arrangement of these atoms within the molecules. One possible explanation for the observed difference is that compound A is an alcohol, specifically ethanol (CH₃CH₂OH), while compound B is an ether, such as dimethyl ether (CH₃OCH₃). The presence of the hydroxyl group (-OH) in ethanol enables it to undergo a reaction with sodium metal, known as the metal-acid reaction. In this reaction, the metal displaces the hydrogen from the hydroxyl group, forming sodium ethoxide (CH₃CH₂ONa) and releasing hydrogen gas (H₂). On the other hand, ethers like dimethyl ether lack the hydroxyl group and therefore cannot undergo the metal-acid reaction. Consequently, when compound B is treated with sodium metal, no hydrogen gas is released. The ability of compound A to release hydrogen gas while compound B does not when treated with sodium metal can be attributed to the presence of a hydroxyl group in compound A (ethanol), enabling it to undergo a metal-acid reaction, whereas compound B (dimethyl ether) lacks the necessary functional group and thus does not undergo this reaction.
For such more questions on structural
https://brainly.com/question/29117530
#SPJ11
Using the following reaction, determine the theoretical yield of Acetylsalicylic acid if given 2.31 grams of salicylic acid? (reminder, salicylic acid is the limiting reagent)
Answers
The theoretical yield of Acetylsalicylic acid is found out to be: 3.01 grams.
What is limiting reagent?The limiting reagent is a substance that prevents a chemical reaction from occurring completely.
When a limiting reagent is used in a chemical reaction, the atoms, molecules, or ions of the other reactant that it (the limiting reagent) reacts with will either stay free or unreacted.
What is acid?Popular compounds called acids and bases interact with one another to create salt and water. The Latin word "acere," which meaning "sour," is where the term "acid" originates.
According to the problem, we have 2.31 grams of salicylic acid. We need to determine the theoretical yield of acetylsalicylic acid.
The molar mass of salicylic acid is 138.12 g/mol. Therefore, the number of moles of salicylic acid we have is:
n = mass / molar mass
n = 2.31 g / 138.12 g/mol
n = 0.0167 mol
According to the balanced equation, 1 mole of salicylic acid reacts with 1 mole of acetic anhydride to produce 1 mole of acetylsalicylic acid. Therefore, the number of moles of acetylsalicylic acid produced is also 0.0167 mol.
The molar mass of acetylsalicylic acid is 180.16 g/mol. Therefore, the theoretical yield of acetylsalicylic acid is:
mass = n x molar mass
mass = 0.0167 mol x 180.16 g/mol
mass = 3.01 g
Therefore, the theoretical yield of acetylsalicylic acid is 3.01 grams.
To know more about reagent visit:
https://brainly.com/question/31228572
#SPJ1
Mercury may have ice trapped in the crater floors near the planet’s north pole.
a. True
b. False
Answers
It’s a test and only need 2 more problems!!!! Thank you and Appreciate it!!!

Answers
Answer:
the molar mass of propane (c3H8) is
12*3+1*8
=36+8=44
Ni
Express your answer in condensed form in the order of orbital filling as a string without blank space between orbitals. For example, [He]2s22p2 should be entered as [He]2s^22p^2.

Answers
Answer:
[Ar]3d^84s^2
Explanation:
From the question given, we are asked to write the condensed form of electronic configuration of nickel, Ni.
To do this, we simply write the symbol of the noble gas element before Ni in a squared bracket followed by the remaining electrons to make up the atomic number of Ni.
This is illustrated below:
The atomic number of Ni is 28.
The noble gas before Ni is Argon, Ar.
Therefore, the condensed electronic configuration of Ni is written as:
Ni(28) => [Ar]3d^84s^2
Answer:
[Ar] 4s^23d^8
Explanation:
Glucose is made up of hydrogen, oxygen, and carbon atoms.
What evidence shows that the atoms that make up glucose have different properties than the molecule glucose?
Answers
Answer:
https://brainly.com/question/11792047
Glucose contains hydrogen atoms from water or H₂O. Carbon dioxide and water are the reactants in the chemical reaction of photosynthesis. Oxygen and the carbon that glucose needs are both provided by carbon dioxide.
Because glucose cannot be further hydrolyzed, it is categorized as a monosaccharide. Due to its six-carbon structure and the presence of an aldehyde group on carbon 1, it is also categorized as an aldose and a hexose.
A glucose molecule is too large to pass through a cell membrane. cells assist glucose diffusion through facilitated diffusion and two types of active transport. This is evidence that the atoms that make up glucose have different properties than the molecule glucose.
To know more about glucose, visit;
https://brainly.com/question/30625394
#SPJ3
Please help asap!! Need help for problem #2.
Naturally occuring iron, contains 5.82% ^54Fe, 91.66% ^56Fe, 2.19% ^57Fe, and 0.33% ^58Fe. The reslective atomic masses are 53.940 amu, 55.935 amu, 56.935 amu and 57.933 amu. Calculate the average atmoic mass of iron. (show work)

Answers
Answer:
55.56 amu
Explanation:
Let A, B, C and D represent the four isotopes of iron.
The following data were obtained from the question:
Isotope A (Fe-54):
Mass of A = 53.940 amu
Abundance (A%) = 5.82%
Isotope B (Fe-56):
Mass of B = 55.935 amu
Abundance (B%) = 91.66%
Isotope C (Fe-57):
Mass of C = 56.935 amu
Abundance (C%) = 2.19%
Isotope D (Fe-58):
Mass of D = 57.933 amu
Abundance (D%) = 0.33%
Average atomic mass =.?
The average atomic mass of the iron can obtained as follow:
Average atomic mass = [(mass of A × A%)/100] + [(mass of B × B%)/100] + [(mass of C × C%)/100] + [(mass of D × D%)/100]
Average atomic mass = [(53.940 × 5.82)/100] + [(55.935 × 91.66 )/100] + [(56.935 × 2.19)/100] + [(57.933 × 0.33)/100]
= 2.848 + 51.270 + 1.247 + 0.191
= 55.56 amu
Therefore, the average atomic mass of the iron is 55.56 amu
Which refrigerant is known to destroy ozone?
ammonia
Freon®
tetrafluoroethane
ice
Answers
Answer:
tetrafluoroethane
Explanation:
Its an HCFC, which are the refrigerants that destroy the ozone layer.
Answer:
Freon.
Explanation:
Brainliest plz???
Classify the following as either an element, compound, homogeneous mixture or
heterogeneous mixture:
milk
Answers
Answer:
HeterogeneousExplanation:
Milk seems to be homogeneous mixture but actually milk is a heterogeneous mixture and a colloid solution.Calculate the average atomic mass of sulfur.

Answers
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances.
avg. atomic mass=∑i(isotopei×abundancei)
atomic masses of four isotopes of sulphur are
32S: 31.972 u→95.002%
33S: 32.971 u→0.76%
34S: 33.967 u→4.22%
36S: 35.967 u→0.014%
the average atomic mass of sulfur will be
Average atomic mass=31.972 u×0.95002+32.971 u×0.0076+33.967 u×0.0422+35.967 u×0.00014
Average atomic mass=32.063 u
Application 2:
1. Another component of acid rain is nitric acid, which forms when NO, also a pollutant, reacts with oxygen and water according to the simplified equation:
4 NO2(g) + 02(g) + 2 H0(1) -> 4 HNO, (ag)
The generation of the electricity used by a medium-sized home produces about 16 kg of NO2 per year. Assuming that there is adequate Oz and H2O, what mass of HNO;, in kg, can form from this amount of NO2 pollutant?

Answers
Chemical reaction in balance: 4HNO3 is the result of 4NO2 + O2 + 2H2O. N has a mass of 14 u, while O has a mass of 16 u.
Which of the following describes a mole in the most comprehensive and accurate manner?The quantity of a substance that contains as many particles as there are atoms in precisely 12 g of carbon 12 isotope is referred to as a mole. Regardless of their nature, 6.0221023 particles are represented by a mole. As a result, when propane is burned, it produces 4 moles of water and 3 moles of carbon dioxide.
When propane C3H8 burns, what kinds of products are produced?Answer: The hydrocarbon propane (C3H8) is a member of the homologous series of alkanes. As a result, propane will be combusted to produce four molecules of water and three molecules of carbon dioxide.
To know more about Chemical reaction visit :-
https://brainly.com/question/29039149
#SPJ1
Nitrogen-13 has a half-life of 20 minutes. how much of a 100 mg sample would remain after 60 minutes?
Answers
The amount of nitrogen-13 sample that remained after 60 minutes has been 25mg.
Half-life can be described as the time required by the substance to reduce half of its initial concentration.
The half-life of Nitrogen-13 has been 20 minutes. In 20 minutes, the sample will be reduced to half of its concentration,
The total time has been 60 minutes.
The number of half-life experienced by the sample has:
Number of half life= Total time/half life
Number of half life cycles= 60/20=3
The number of half-life cycles = 3
The sample has been reduced to 50% in the first half-life cycle and reduced to 25% by the end of 2nd half-life cycle.
The sample remained = 25% of the initial concentration.
The sample remained = 25/100.100 mg
The sample remained = 25 mg
Therefore, the amount of nitrogen-13 sample that remained after 60 minutes has been 25mg.
To learn more about half-life from the given link.
https://brainly.com/question/1160651
#SPJ1
An early arrangement of the then known elements was proposed by a British scientist John Newlands, which he called the Law of Octaves. Like other scientists at the time, Newlands arranged the elements in order of increasing atomic mass and noted that every eighth element had similar physical/chemical properties. In the modern Periodic Table, which of the following represents the last pair of elements for which Newlands' Law of Octaves would hold true?
Answers
An error during which cellular process would create a gene mutation?
Answers
An error during DNA replication would create a gene mutation.
During DNA replication, the genetic information in a cell is copied to make new DNA molecules. However, mistakes can occur during this process, leading to changes in the DNA sequence, which can result in a mutation. Mutations can also be caused by exposure to environmental factors, such as radiation or chemicals, which can damage the DNA molecule directly or affect the cellular processes involved in DNA replication.
Mutations can have a variety of effects on the organism, ranging from no effect to causing serious health problems or even death. Gene mutations can also be inherited from a parent, which can result in genetic disorders or predisposition to certain diseases. Therefore, it is important to understand the mechanisms of gene mutations and their potential impacts on organisms.
To know more about the Gene mutation, here
https://brainly.com/question/15448555
#SPJ1
How much grams are in 4.78 cup
Answers
Answer:
1130.89
Explanation:
Answer:
963.648
Explanat:::::::)))))))
When a 17.9 mL sample of a 0.458 M aqueous nitrous acid solution is titrated with a 0.368 M aqueous potassium hydroxide solution, what is the pH after 33.4 mL of potassium hydroxide have been added
Answers
Answer:
pH = 12.90
Explanation:
THe reaction of HNO₃ with KOH is:
HNO₂ + KOH → KNO₂ + H₂O
That means 1 mole of nitrous acid reacts per mole of potassium hydroxide.
To solve this question, we need to find the moles of each reactant:
Moles HNO₂:
0.0179L * (0.458mol / L) = 0.00820 moles
Moles KOH:
0.0334L * (0.368mol / L) = 0.01229 moles
That means KOH is in excess. The moles in excess are:
0.01229 moles - 0.00820 moles = 0.00409 moles KOH = Moles OH⁻
The [OH⁻] is -Total volume = 17.9mL+33.4mL = 51.3mL = 0.0513L-:
0.00409 moles / 0.0513L =
0.0797M =[OH⁻]
pOH = -log[OH⁻] = 1.098
pH = 14 - pOH
pH = 12.90Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
A nitrogen-containing compound shows no absorption band at ∼3400cm−1 and no absorption bands between ∼1700cm−1 and ∼1600cm−1. what class of compound is it
Answers
Explanation:
A nitrogen-containing compound that shows no absorption band at around 3400 cm^−1 and no absorption bands between approximately 1700 cm^−1 and 1600 cm^−1 is likely an amide compound.
Amides typically exhibit a characteristic absorption band in the region of 3200-3500 cm^−1 due to the N-H stretching vibration. The absence of this absorption band suggests the absence of N-H bonds, which rules out compounds like primary or secondary amines.
The absence of absorption bands between 1700 cm^−1 and 1600 cm^−1 eliminates functional groups such as carbonyl compounds (e.g., aldehydes, ketones, carboxylic acids, esters) and imines, which typically exhibit absorption in this region.
Therefore, based on the given information, it can be inferred that the compound is likely not an amine, carbonyl compound, or imine. Other classes of compounds that do not possess these characteristic absorption bands would need to be considered.
An American visiting Canada puts 41.2 liters of gas in his car. How much is that in gallons? (1 gal = 3.78 L)
Answers
Answer:
41.2/3.78=10.9
(this is rounded off to the first decimal)
Answer:
10.9 gal
Explanation:
Recall that 1gal = 3.78l
if 1gal = 3.78l
then Xgal = 41.2l
cross multiplywe have 1gal*41.2L=Xgal* 3.78L
divide both by 3.78L1gal*41.2L/3.78= Xgal
Xgal = 10.89L or 10.9L