3-Chloro-1-pentene reacts with sodium ethoxide in ethanol to produce 3-ethoxy-1-pentene. The reaction is second order, first order in 3-chloro-1-pentene and first order in sodium ethoxide. In the absence of sodium ethoxide, 3-chloro-1-pentene reacts with ethanol to produce both 3-ethoxy-1-pentene and 1-ethoxy-2-pentene. The first reaction proceeds via an mechanism. The stereochemistry of the product is . The second reaction proceeds via an mechanism. The stereochemistry of 3-ethoxy-1-pentene is . The stereochemistry of 1-ethoxy-2-pentene is . For the second reaction, draw the structure of the intermediate's resonance contributor that leads to the formation of 3-ethoxy-1-pentene.
Answers
Answer:
The first case is an SN2 reaction while the second case is an SN1 reaction
Explanation:
In the first case, what we have is an SN2 bimolecular reaction. It should be recalled that sodium ethoxide is a strong nucleophile. Strong nucleophiles lead to SN2 substitution in secondary alkyl halides where SN1 or SN2 mechanisms are probable. The observed mechanism now depends on the actual nucleophile used in the reaction. Since a strong nucleophile is used, 3-ethoxy-1-pentene is obtained as shown in the image attached to this answer.
In the second case, a weaker nucleophile, ethanol is used. The use of a weak nucleophile leads to an SN1 mechanism. The 3-Chloro-1-pentene forms a secondary carbocation as shown in the image attached. This secondary carbocation can re-arrange itself to give the two products; 3-ethoxy-1-pentene and 1-ethoxy-2-pentene as shown in the reaction mechanism in the image attached. The structure of the contributor leading to the formation of 3-ethoxy-1-pentene is clearly shown in the image attached.

Related Questions
Throughout the reflection, make sure you have a copy of the Student Guide and your data tables. Use the drop-
down menus to complete the statements.
The independent variable, the one that is intentionally manipulated, is
The dependent variable, the one that you measure the response in, is the pH of the solution
the physical properties
the system for measurement
Answers
Answer:
1) mass and type of material
2) type of material
3) temperature
Explanation:
Answer:
For Ed
Explanation:
The first one is: The physical proprieties.
The second one is: The pH of the solution.
I've been seeing the same answers from above around though, so I may be wrong. But either way, I tried.
The atomic number of an atom is equal to the number of protons electrons neutrons a.protons b.electrons c.neutrons
Answers
Answer:
A
Explanation:
Atomics number = number of protons
what is the last element in the periodic table?
Answers
Answer:
Ununoctium
Explanation:
It's at the very end of the periodic table
Answer:
Ununoctium
Explanation:
Ununoctium is the last chemical element int the periodic table.
Plz help!!!! Solve this by using factor labeling

Answers
Answer:
the answer is 2,000 nickels.
Explanation:
we multiplied 100 by 100, because there are 100 cents in a dollar, and we divided 10,000 by 5, because there are 5 cents in a nickel.
In pic first balence and then solve question Part a.

Answers
Answer:
42.1 g of silver chloride (AgCl).
Explanation:
What is given?
Mass of silver nitrate (AgNO3) = 50.0 g.
Molar mass of silver nitrate (AgNO3) = 170 g/mol. (you can calculate this using the periodic table)
Molar mass of silver chloride (AgCl) = 143.4 g/mol.
Step-by-step solution:
First, let's balance the chemical equation by trial and error method:
\(AgNO_3+BaCl_2\rightarrow AgCl+Ba(NO_3)_2.\)You can see that we have Cl and NO3 unbalanced, so if we put '2' moles beside AgCl, chlorine (Cl) will be balanced for both sides, but Ag would be unbalanced and NO3 too, so if we put '2' moles beside AgNO3, we will obtain the balanced equation:
\(2AgNO_3+BaCl_2\operatorname{\rightarrow}2AgCl+Ba(NO_3)_2\)Now, let's do the stoichiometry of part a.
Let's convert 50.0 g of AgNO3 to moles using its given molar mass:
\(50.0\text{ g AgNO}_3\cdot\frac{1\text{ mol AgNO}_3}{170\text{ g AgNO}_3}=0.294\text{ moles AgNO}_3.\)And now, let's see how many moles of AgCl are being produced by 0.294 moles of AgNO3. You can see in the chemical equation that 2 moles of AgNO3 reacted, produces 2 moles of AgCl, so the molar ratio is 2:2, more simply is 1:1. This means that if we're using 0.294 moles of AgNO3, we will obtain 0.294 moles of AgCl.
Based on this logic, the final step is to find the mass of AgCl in grams, using its molar mass, like this:
\(0.294\text{ moles AgCl}\cdot\frac{143.4\text{ g AgCl}}{1\text{ mol AgCl}}=42.1\text{ g AgCl.}\)The answer is we will obtain 42.1 g of silver chloride (AgCl).
Consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. The resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. If, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. An actual vapor pressure greater than that predicted by Raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by Raoult's law is a negative deviation.
Part A
Imagine a solution of ftwo liquids in which the molecules interact less favorably than they do in the individual liquids Will this solution deviate posltively from, deviate negatively from, or idealy follow Raouit's law?
a. It will deviate positively.
b. It will deviate negatively.
c. It will be an ideal solution
Part B
Imagine a solution of two iquids in which the molecules interact more favorably than they do in the individual liquids Will this solution deviate positively from, deviate negatively from, or ideally follow Raoult's law?
a. It will deviate positively. b. It will deviate negatively. c. It will be an ideal solution
Answers
Answer:
b. a.
Explanation:
A homogenous mixture varies from a heterogenous mixture in that a homogenous mixture has properties that:A) do not vary in the mixtureB) vary in the mixtureC) suspend in a liquidD) dissolve a solute
Answers
A homogenous mixture varies from a heterogenous mixture in that a homogenous mixture has properties that do not vary in the mixture. The correct answer is A.
A homogeneous mixture is a mixture in which the properties and composition are uniform throughout the mixture. This means that if you took a sample from any part of the mixture, you would find the same properties and composition in that sample as you would find in the entire mixture.
In contrast, a heterogeneous mixture is a mixture in which the properties and composition vary throughout the mixture. For example, a salad is a heterogeneous mixture because you can see different ingredients such as lettuce, tomatoes, and croutons, each with different properties and composition.
So, in a homogeneous mixture, the properties do not vary, while in a heterogeneous mixture, the properties vary throughout the mixture.
Learn more about homogenous mixture here: https://brainly.com/question/1307383
#SPJ4
Which compound contains ionic bonds?
1.
N20
2.
Na20
3.
СО
4.
CO2
Answers
To find the order of a reaction with respect to one reactant, you will monitor the as the of . is changed.
Answers
The order of reaction is defined as the power to which the concentration of the reactants are raised in the rate equation of the reaction.
The order of reaction can be used to determine how a particular reactant affects the reaction. In order to find the order of a reaction with respect to a particular reactant, the concentration of the reactant is changed while keeping the concentration of other reactants constant. The rate of reaction is then measured and compared with the rate of reaction when the concentration of the reactant is not changed.The order of reaction with respect to a reactant can be determined using the following method:First, select a reactant whose order needs to be determined and change its concentration while keeping the concentration of other reactants constant. For example, if we want to find the order of reaction with respect to reactant A, we will change the concentration of A and keep the concentration of reactant B constant.Second, measure the rate of reaction at different concentrations of the reactant A. The rate of reaction can be measured by any suitable method such as change in color, pH, or by measuring the amount of product formed with time. A graph is plotted with rate of reaction on the y-axis and concentration of reactant A on the x-axis. The graph should be a straight line.Third, if the graph is a straight line passing through the origin, the order of reaction with respect to reactant A is one. If the graph is a straight line but does not pass through the origin, the order of reaction with respect to reactant A is two. If the graph is not a straight line, the order of reaction with respect to reactant A is either zero or fractional.For such more question on concentration
https://brainly.com/question/17206790
#SPJ8
500mg of iron(ll) complex ferrous bisglycinate hydrochloride was dissolved in dilute H2SO4 and titrated with 0.0200 mol. dm3 KMnO4 18.10cm3 of KMnO4 solution were require to reach the end point the equation for the titration reaction is as follows 5Fe2+ + MnO4- + 8H+ - - > 5Fe2+ + Mn2+ +4H2O
Calculate the
1.number of moles of Fe2+ in the capsule
2.mass of iron in the capsul
3.molar mass of the iron (ll) complex assuming 1mole of the complex contains 1mole of iron. (Fe=55.9gmol-1)
Answers
Using the stoichiometry of the reaction, we can obtain the molar mass of the complex as 276 g/mol. The number of moles iron II as 0.00181 moles and the mass of iron II as 0.1 g.
Redox reactionA redox reaction is one in which there is loss or gain of electrons. The equation of the reaction is 5Fe2+ + MnO4- + 8H+ - - > 5Fe2+ + Mn2+ +4H2O
Number of moles of permanganate = 0.0200 mol. dm3 * 18.10cm3/1000 L
= 0.000362 moles
If 5 moles of iron II reacted with 1 mole of permanganate
x moles of iron II reacts with 0.000362 moles of permanganate
x = 5 moles * 0.000362 moles/ 1 mole
= 0.00181 moles of iron II
Recall that;
Number of moles = mass/molar mass
Molar mass of iron II = 56 g/mol
0.00181 moles = x/56 g/mol
x = 0.00181 moles * 56 g/mol = 0.1 g
If 1 mole of the complex contains 1 mole of iron then;
0.00181 moles = 500 * 10^-3/MM
MM= 500 * 10^-3/0.00181 moles
MM = 276 g/mol
Learn more about stoichiometry: https://brainly.com/question/9743981
What is the mass, in grams, of 1.16 mol of water, H2O?
Answers
The mass, in grams of 1.16 mole of water, H₂O is 20.88 g
Description of moleThe mole of a substance is related to it's mass and molar mass according to the following equation
Mole = mass / molar mass
With the above formula, we can obtain the mass of H₂O
Data obtained From the questionFrom the question given above, the following data were obtained:
Mole of H₂O = 1.16 mole Molar mass of H₂O = (2×1) + 16 = 18 g/mol Mass of H₂O =? How to determine the mass of H₂OThe mass of 1.16 mole of water can be obtained as illustrated below:
Mole = mass / molar mass
Cross multiply
Mass = mole × molar mass
Mass of H₂O = 1.16 × 18
Mass of H₂O = 20.88 g
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
how many moles of pbcl2 are produced when 16 alcl3 are consumed
Answers
How many moles of titanium (Ti) atoms are in 85.3 g of Ti?
Answers
Answer:
1.7820210165667731 your welcome :)
Explanation:
A gas is in a sealed container. How do you think the pressure will change if the container is cooled. explain your answer
Answers
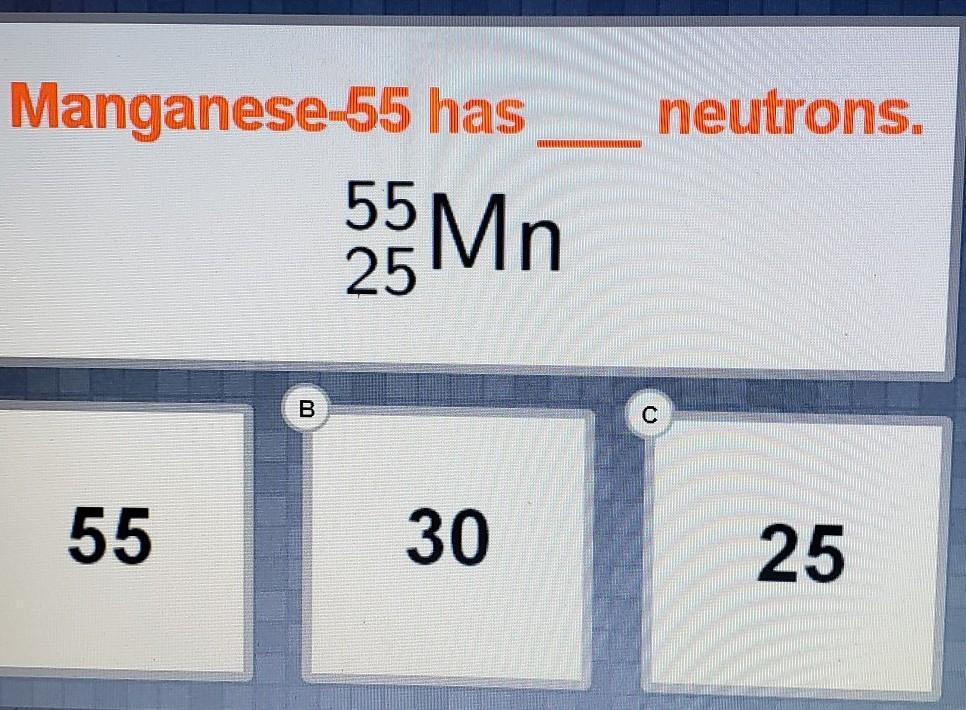
Manganese-55 has _____neutrons.
55 Mn
25
A. 55
B. 30
C. 25
Answers
QUESTION:- Manganese-55 has _____neutrons.
OPTIONS :-
A. 55
B. 30
C. 25
ANSWER:- NUMBER OF NEUTRONS IS EQUAL TO THE DIFFERENCE BETWEEN THE MASS IF THE ATOM AND ATOMIC NUMBER
SO DIFFERENCE IS EQUAL TO :- 55-25 = 30 NEUTRONS.
SO THERE IS 30 NEUTRONS IN SINGLE ATOM OF THE MANGANESE-55 ATOM.
Answer:
the mass of an atom is the sum of proton and neutron which are both concentrated in nocleus of an atom. from the question the mass is given as 55 and the proton is 25.
What is different types of
Metal
Answers
Answer:
copper metal and steel metak
Answer:
Every element to the left of the staircase on the Periodic Table is a metal, excluding Hydrogen, and everything to the right of that staircase is a non-metal.
Explanation:
In which of the following sets is the symbol of the element, the number of protons, and the number of electrons given correctly?
A. In, 49 protons, 49 electrons
B. Zn, 30 protons, 60 electrons
C. Cs, 55 protons, 132.9 electrons
D. F, 19 protons, 19 electrons
Answers
Answer:
A.
Explanation:
The number of the them will result in A.
do anyone know how to do question B

Answers
Answer:
a) IUPAC Names:
1) (trans)-but-2-ene
2) (cis)-but-2-ene
3) but-1-ene
b) Balance Equation:
C₄H₁₀O + H₃PO₄ → C₄H₈ + H₂O + H₃PO₄
As H₃PO₄ is catalyst and remains unchanged so we can also write as,
C₄H₁₀O → C₄H₈ + H₂O
c) Rule:
When more than one alkene products are possible then the one thermodynamically stable is favored. Thermodynamically more substituted alkenes are stable. Furthermore, trans alkenes are more stable than cis alkenes. Hence, in our case the major product is trans alkene followed by cis. The minor alkene is the 1-butene as it is less substituted.
d) C is not Geometrical Isomer:
For any alkene to demonstrate geometrical isomerism it is important that there must be two different geminal substituents attached to both carbon atoms. In 1-butene one carbon has same geminal substituents (i.e H atoms). Hence, it can not give geometrical isomers.

is oil and water heterogeneous or homogeneous?
Please i need this answer for my online class
Answers
Answer:
They are examples of heterogeneous
Explanation:
By definition, a pure substance or a homogeneous mixture consists of a single phase. A heterogeneous mixture consists of two or more phases. When oil and water are combined, they do not mix evenly, but instead form two separate layers.
Find the mass in 34.4 liters of O2 gas at STP?
Answers
Answer:
mass of 1 mole of 34.4liters, O2, STP = 95.039999g
Explanation:
Write the problem as a mathematical expression.
Find the mass in 34.4liters of O2 gas at STP
To find the mass of 1 mole of 34.4liters, O2, STP look up the atomic mass of each element and multiply it by the number of atoms contained in each element in the molecule.
mass of 1 mole of 34.4liters, O2, STP = 2(mass of O) + (mass of S) + (mass of T) + (mass of P)
Fill in the atomic masses from the periodic table.
mass of 1 mole of 34.4liters, O2, STP = 2(16) + (32.06999969) + (0) + (30.96999931)
Simplify the result.
mass of 1 mole of 34.4liters, O2, STP = 95.039999g
hope this helps, good luck :)
also ive got no clue why the spacing looks all funny, sry about that
Use the ball and stick models above. If you had a gram of water and a gram of
oxygen, which substance would you have more particles of? Why? (Right or Wrong)
Answers
Answer:
When the weather is nice, many people begin to work on their yards and homes. For many projects, sand is needed as a foundation for a walk or to add to other materials. You could order up twenty million grains of sand and have people really stare at you. You could order by the pound, but that takes a lot of time weighing out. The best bet is to order by the yard, meaning a cubic yard. The loader can easily scoop up what you need and put it directly in your truck.
Avogadro’s Number
It certainly is easy to count bananas or to count elephants (as long as you stay out of their way). However, you would be counting grains of sugar from your sugar canister for a long, long time. Atoms and molecules are extremely small – far, far smaller than grains of sugar. Counting atoms or molecules is not only unwise, it is absolutely impossible. One drop of water contains about 10 22 molecules of water. If you counted 10 molecules every second for 50 years without stopping you would have counted only 1.6 × 10 10 molecules. Put another way, at that counting rate, it would take you over 30 trillion years to count the water molecules in one tiny drop.
Explanation:
Which is one way that Earth's atmosphere supports life?
It filters out oxygen
It keeps Earth warm.
It increases UV radiation.
It provides a solid shield.
Answers
Answer:
The answer is B
Explanation:
Sorry if am wrong.
Option B is the correct option. Earth's atmosphere supports life by keeping the earth warm.
Green House Effect:
The gases present in the atmosphere of the earth trap the heat energy sunlight and make the earth warmer. Without an atmosphere, the Earth reflects the whole sunlight and becomes an ice ball.
Atmosphere keeps the earth warm.It helps to protect us from uv rays.Oxygen is not filtered by the atmosphere.Therefore, option B is the correct option. Earth's atmosphere supports life by keeping the earth warm.
To know more about the atmosphere, refer to the link:
https://brainly.com/question/24092645
If the difference between 30 tines k and 35 is 235 find the value of k
Answers
30k-35=235 so 30k= 235+35
30k= 270
K=9
1.20 × 104) × (2.152 × 102) = × 10^6 203/5.3=
Answers
I hoped that helped by the way I went on https://doyourmath.com/web-algebrator/#c=solve&v1=1.2%255C%2520x%255C%2520104%255C%2520x%255C%25202.152%255C%2520x%255C%2520102%253D10%255E%257B6%255C%2520%257D%255Cleft%2528%255Cfrac%257B203%257D%257B5.3%257D%255Cright%2529%255Cnl%2520&v2=solve&v3=x To get the answer.

The size and strength of a volcanic eruption is called
a. magnitude b. degree c. explosive power d. blast radius
Answers
Answer:
The Volcanic Explosivity Index (VEI) is a relative measure of the explosiveness of volcanic eruptions. It was devised by Chris Newhall of the United States Geological Survey and Stephen Self at the University of Hawaii in 1982. ... The scale is open-ended with the largest eruptions in history given a magnitude of 8.
Explanation:
A hypothesis must be testable. Which hypothesis is testable? *
Ablue is the best color.
BSummer is nicer than fall.
CDogs are better than cats.
DA beagle can jump higher than a Persian cat.
Answers
the answer is D because can be tested the others ones is opinion
please mark me as brainliest
What is the chemical formula for the ionic compound formed by Na+ and N¯³?
Answers
Answer:
Na3N
Explanation:
cuzdoodlikeimsosure
In a sack of marbles there are red marbles each weighing 11.50g and green marbles which each weighing 12.70g. There are 10,051 red marbles and 24,351 green marbles. Determine the mass of the average marble in the sack. (Explain why the answer is not equal to (11.50 + 12.70) / 2 = 12.10g .
Answers
The mass of the average marble in the sack is 12.35 g.
Total number of marbles in the bag = number of red marbles + number of green marbles
Mass of each green marble = 12.70g
Mass of each red marble = 11.50g
Total mass of green marbles = 24,351(12.70g) = 309257.70 g
Total mass of red marbles = 10,051(11.50g) = 115586.50 g
Total mass of marbles in the sack = 309257.70 g + 115586.5 g = 424844.22 g
Total number of marbles in the sack = 24,351 + 10,051 = 34402 marbles
Therefore;
Mass of the average marble in the sack = 424844.22 g/34402 marbles
= 12.35 g
Hence, the mass of the average marble in the sack is 12.35 g
Learn more: https://brainly.com/question/178217
A car with a mass of 2000 kg accelerates at a rate of 3 m/s2. How much force does this car have?
Answers
Answer:
6000N
Explanation:
f= ma
f= 2000kg×3mls2
f=6000N
Answer:
6000 N
Explanation:
Given,
Mass= 2000 kg
Acceleration= 3 m/s²
Force= ?
Force= mass* acceleration
Force= 2000* 3 = 6000 N
Therefore, the force possessed by the car is 6000 N
Write the chemical formula for 1 mol of compound X containing 2 mol Al and 3 mol O
Answers
The chemical formula has been defined as the representation of the chemical symbols along with the coefficient and proportions. The chemical formula of the unknown compound X is Al₂O₃.
What is the chemical formula?The chemical formula has been the representation of the relative numbers of the elemental atoms involved in the chemical substance that makes the molecule and the compound. The chemical formula of the unknown substance can be calculated from the number of moles.
The moles of the atom or the element represent the proportions of the atom involved in the various elements in the substance. The chemical formula is represented by letters, coefficients, subscripts, superscripts, parentheses, signs, etc.
Given,
Moles of compound X = 1 mol
Moles of Al element = 2 mol
Moles of O element = 3 mol
So the chemical formula of the unknown compound X will be Al₂O₃ as there are two moles of aluminum and three moles of oxygen in the compound.
Therefore, Al₂O₃ is the chemical formula of the unknown compound X.
Learn more about chemical formulas, here:
https://brainly.com/question/16950183
#SPJ2