Your friend who is using a recipe for flan from mexican cookbook. You notice that he set your oven temperature at 175 fahrenheit. What would you advise him to do
Answers
If your friend who is using a recipe for flan from a Mexican cookbook and you notice that he set your oven temperature at 175 Fahrenheit, advise him to raise the oven temperature to 350 Fahrenheit.
Flan is a Mexican dessert that is similar to crème caramel. It is made by baking a mixture of sugar, milk, eggs, and vanilla in a water bath until it is set. If your friend who is using a recipe for flan from a Mexican cookbook and you notice that he set your oven temperature at 175 Fahrenheit,
advise him to raise the oven temperature to 350 Fahrenheit.A temperature of 175 Fahrenheit is too low to bake flan. At this temperature, the flan will take too long to cook, and the result will be a runny, unappetizing mess. Flan should be baked at a temperature of around 350 Fahrenheit, which is hot enough to cook the flan through quickly but not so hot that it burns or becomes overcooked. At this temperature, the flan will be creamy, rich, and delicious. Therefore, it's recommended to raise the oven temperature to 350 Fahrenheit.
TO know more about that temperature visit:
https://brainly.com/question/7510619
#SPJ11
Related Questions
Summarize the process of anabolism.
Answers
The process of anabolism is a metabolic pathway in which one or more molecules are used to synthesize more complex compounds.
What is anabolism?Anabolism refers to the chemical metabolic reactions that occur in biological systems which are aimed at generating complex molecules from simpler ones. This process is different from catabolism, which involves the release of energy to be used in the anabolic process.
Therefore, with this data, we can see that anabolism refers to the generation of complex molecules in biological systems by using the energy generated by catabolic reactions in the body.
Learn more about anabolism here:
https://brainly.com/question/25970390
#SPJ1
Calculate the pH of the solution that results from each of the following mixtures.Part A50.0mL of 0.16M HCHO2 with 80.0mL of 0.11M NaCHO2Express your answer using two decimal places.
Answers
The pH of the resulting solution is 3.78.
To calculate the pH of the resulting solution from mixing 50.0 mL of 0.16 M HCHO2 with 80.0 mL of 0.11 M NaCHO2, we can use the Henderson-Hasselbalch equation:
pH = pKa + log ([A-]/[HA])
First, we need to find the moles of HCHO2 and NaCHO2:
moles of HCHO2 = 0.16 M × 0.050
L = 0.008 mol moles of NaCHO2 = 0.11 M × 0.080
L = 0.0088 mol
Next, we determine the final concentrations of HCHO2 and NaCHO2 after mixing:
[A-] = moles of NaCHO2 / (0.050 L + 0.080 L) = 0.0088 mol / 0.130
L = 0.0677 M [HA] = moles of HCHO2 / (0.050 L + 0.080 L) = 0.008 mol / 0.130
L = 0.0615 M
Now we need the pKa of HCHO2, which is 3.74. We can plug these values into the Henderson-Hasselbalch equation:
pH = 3.74 + log (0.0677 M / 0.0615 M)
pH = 3.74 + 0.037 pH = 3.78 (rounded to two decimal places)
Learn more about pH at https://brainly.com/question/29557587
#SPJ11
Shown below are the structures of sulfuric acid, H2SO4, and sulfate citing evidence from Part A. bonds in nm. Does sulfate exist as a bonds in nm. Does sulfate exist as a resonance hybrid? What's the evidece? iig the lengths of 098 nm 63 nm 45 nm 45 153 ro 153 nm nm 53 nm 098 nm Sulfuric Acid Sulfate
Answers
Yes, sulfate (SO₄²⁻) exists as a resonance hybrid.
The evidence for this is the equal bond lengths between the sulfur atom and the four oxygen atoms in the sulfate ion. Due to resonance, electrons are delocalized across the entire ion, resulting in an even distribution of electron density and equal bond lengths.
In the case of sulfate ion, it can be represented by two resonance structures, as follows:
O=S(=O)(-O⁻) and O(-S(=O)(=O))-O⁻
In the first resonance structure, the sulfur atom has a double bond with one oxygen atom and a single bond with two other oxygen atoms, while in the second resonance structure, the sulfur atom has a double bond with two oxygen atoms and a single bond with one other oxygen atom.
These two resonance structures have the same arrangement of atoms, but the placement of electrons is different.
Due to resonance, the electrons in the sulfate ion are delocalized across the entire ion. This results in an even distribution of electron density and equal bond lengths between the sulfur atom and the four oxygen atoms in the sulfate ion.
The bond lengths between sulfur and oxygen atoms are all about 143 pm, which is intermediate between the typical bond lengths of double and single bonds. This is consistent with the idea that the electrons are delocalized between the two resonance structures.
To learn more about resonance hybrid, refer below:
https://brainly.com/question/28318319
#SPJ11
What term do we use to describe how bright a star “seems” to us here on Earth?
absolute magnitude
apparent magnitud
Answers
Answer:
Apparent magnitude
Explanation:
By definition, Apparent magnitude which is usually denoted by "m" is the measure of the brightness of a star or even other astronomical objects that are observed from Earth. Meanwhile the absolute magnitude is defined as the intrinsic luminosity emitted by a star or other astronomical objects.
Thus, apparent magnitude is correct.
A 10 kg package is delivered to your house.
Use one complete sentence to describe an example of how work is done on the package as it gets brought inside.
Make sure to use proper spelling, grammar, and other language mechanics.
In your explanation, make sure to use the terms related to the formula for work (W = Fd).
Answers
Answer:
Explanation:
Work is a net force applied through a distance in order to displace an object, commonly abbreviated as W. A net force is the sum of all forces acting on an object. Work is mass times acceleration and distance so to find out the work you simply calculate the acceleration of the box being brought in. Next find the distance it was carried to get in the house. Then find out the mass of the box and finally multiply those sums together to get the amount of work put in to bring the package inside.
How much Naclo3 will dissolve at 50°C?
Answers
Answer:
90.
Explanation: yea and I will be back at your
Guided
Learning
ar Inequalities in One Variable - Item 7601
Pre-Quiz
Practice
from a, it will be greater than b.
Post-Quiz
Finish
If the ordered pair (a, b) satisfies the inequality y> x-4, three of these statements are
true. Which statement is NOT true?
a and b may be equal to each other.
We
Answers
The statement about the inequality that is NOT true is (A), a and b may be equal to each other.
How to determine true statements?This is because the inequality y> x-4 means that y must be greater than x-4. If a and b are equal, then y = x-4, which means that the inequality is not satisfied.
The other three statements are true because:
If 4 is subtracted from a, it will be greater than b because y> x-4 means that y must be greater than x.
If 4 is subtracted from b, it will be less than a because y> x-4 means that y must be greater than x.
If 4 is subtracted from both a and b, the inequality will still be true because y> x-4 means that y must be greater than x, even if x and b are both decreased by 4.
Therefore, the answer to the question is the statement a and b may be equal to each other.
Find out more on inequality here: https://brainly.com/question/25275758
#SPJ1
Complete question:
Guided Learning ar Inequalities in One Variable - Item 7601
Pre-Quiz
Practice
from a, it will be greater than b.
Post-Quiz
Finish
If the ordered pair (a, b) satisfies the inequality y> x-4, three of these statements are true. Which statement is NOT true?
a and b may be equal to each other.
If you subtract 4 from a, it will be greater than b.
If you subtract 4 from b, it will be less than a.
If you subtract 4 from both a and b, the inequality will still be true.
Which of the following compounds would form a
crystal lattice?
water
sugar
paraffin wax
copper ID chloride
Answers
Answer:
Water
Explanation:
When water freezes it forms a crystal lattice shape
Copper chloride would form a crystal lattice. Therefore, option D is correct.
What is crystal lattice?In crystallography, a crystal lattice can be described as a description of the ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures take place from the intrinsic nature of the particles to form symmetric that repeat along the principal directions of 3-D space in matter.
The smallest group of atoms in the material that constitutes this repeating pattern is known as the unit cell. The unit cell fully reflects the symmetry and structure of the crystal, which is built up by the repetition of the unit cell along its principal axes.
The lengths of the principal axes of the unit cell and the angles are the lattice constants called lattice parameters. The symmetry properties of the crystal are based on the concept of space groups.
The crystal structure and symmetry determine many physical properties, such as electronic band structure, cleavage, and optical transparency.
Learn more about crystal lattice, here:
https://brainly.com/question/11207173
#SPJ2
what is the mole ratio between ammonia and nitrogen in the above reaction?

Answers
The chemical equation of the production of ammonia is shown in figure 1 and it shows that the mole ratio for ammonia and nitrogen gas is 2:1.
What is mole ratio?
A mole ratio in chemistry is the ratio of the mole quantities of any two chemicals that are involved in a chemical reaction. The balanced chemical equation for the reaction, which displays the proportional numbers of molecules or moles of each reactant and result, is where it is generated. Stoichiometry, or the computation of the amounts of reactants and products in a chemical process, makes use of mole ratios. They can be used to forecast how much of a product will be formed from a given amount of reactant or to figure out how much of one material is required to react completely with a given amount of another substance.To know more about mole ratio, click the link given below:
https://brainly.com/question/15288923
#SPJ1
Science (Grade 8)
What force is always directed opposite to the motion of an object?
A.Friction
B.Gravitational
C.Magnetic
D.Tension
What force pulls an object back to the earth?
A. Applied
B. Gravitational
C.Magnetic
D.Tenstion
What surface would be easiest for a bicycle to move?
A.Sand
B.Grass
C.Muddy Roud
D.Concrete Road
What force acts perpendicular to the surface of the object in contact with?
A. 0 N
B. 300 N
C. 600 N
D. 900 N
What is the net force in the figure bellow?
A.25 N, to the left
B.25 N, to the right
C.195 N, to the left
D.195 N, to the right
Can you plz answer this?
I need it right away...
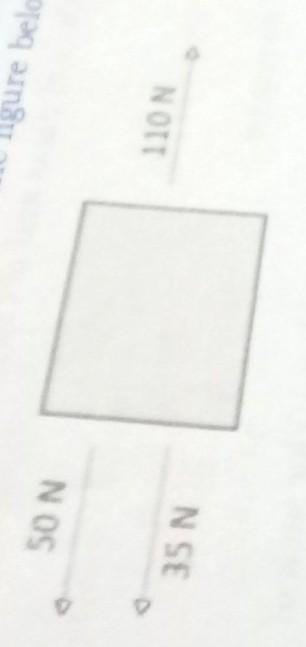
Answers
Answer:
1. Frictional. 2. Gravitational. 3. Concrete road. 4.0N
please help.
what is the relationship between period number and the number of occupied energy levels?
Answers
Answer:
The number ''n''of a period on the periodic table is the same as the number of the highest principal energy level for the atoms on that row that is, the principal energy level occupied by its valence electrons. Thus, elements on period 1 have a highest principal energy level of 1, and so on.
What is the molality of a solution of 30.1 g of propanol (CH3CH2CH2OH) in 152 mL water, if the density of water is 1.00 g/mL
Answers
3.29 mol/kg is the molality of a solution of 30.1 g of propanol (CH3CH2CH2OH) in 152 mL water, if the density of water is 1.00 g/mL
To find the molality of the solution, we first need to calculate the number of moles of propanol and the mass of water in the solution.
1. Calculate the number of moles of propanol:
- The molar mass of propanol (CH3CH2CH2OH) is 60.10 g/mol.
- Divide the mass of propanol (30.1 g) by the molar mass to find the number of moles: 30.1 g / 60.10 g/mol = 0.501 moles.
2. Calculate the mass of water:
- The density of water is 1.00 g/mL.
- Multiply the density by the volume of water (152 mL) to find the mass: 1.00 g/mL * 152 mL = 152 g.
Now, we can calculate the molality using the formula:
Molality (m) = moles of solute / mass of solvent (in kg).
3. Convert the mass of water from grams to kilograms: 152 g / 1000 = 0.152 kg.
4. Calculate the molality: 0.501 moles / 0.152 kg = 3.29 mol/kg.
In conclusion, the molality of the solution is 3.29 mol/kg.
To know more about molality visit:
https://brainly.com/question/30640726
#SPJ11
If 16.4 g of oxygen gas react with excess hydrogen, how many moles of water are produced?
2H2(g) + O2(g) → 2H2O(g)
Answers
Answer:
1.02 (or 1.03 if rounded)
Explanation:
Given 16.4 grams of oxygen gas, you would want to write that down to set up your unit conversions.
16.4 g O2/1 * 1 mol of O2/31.998 g O2 * 2 mol H2O/1 mol O2
Multiply the numbers on the top and divide that by the product of the numbers on the bottom.
16.4 * 1 * 2 = 32.8
1 * 31.998 * 1 = 31.998
32.8/31.998 = 1.025...
Don't forget to consider the number of significant figures if it asks!
A chemical reaction in which two aqueous compounds exchange ions to produce two new compounds is a ______ reaction.combustionelectrolysisdouble-displacement
Answers
The chemical reaction described, where two aqueous compounds exchange ions to produce two new compounds, is a double-displacement reaction.
In a double-displacement reaction, also known as a metathesis reaction or a double replacement reaction, the cations and anions of two different compounds switch places to form new compounds. The reactants are typically in the aqueous phase, meaning they are dissolved in water.
The general format of a double-displacement reaction can be represented as follows:
AB + CD -> AD + CB
Where A, B, C, and D represent different ions or elements.
In this type of reaction, the positive ions (cations) from each compound combine with the negative ions (anions) from the other compound to form two new compounds. The cations and anions essentially swap partners, resulting in the formation of different products.
One classic example of a double-displacement reaction is the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl):
AgNO₃ (aq) + NaCl (aq) -> AgCl (s) + NaNO₃ (aq)
In this reaction, the silver cation (Ag+) from silver nitrate combines with the chloride anion (Cl-) from sodium chloride, resulting in the formation of silver chloride (AgCl) as a solid precipitate. Simultaneously, the sodium cation (Na+) from sodium chloride combines with the nitrate anion (NO₃⁻) from silver nitrate to produce sodium nitrate (NaNO₃) in the aqueous phase.
Double-displacement reactions are common in various chemical processes and can result in the formation of precipitates, gases, or other products. They often occur in aqueous solutions and can be used to identify the presence of certain ions or compounds through the formation of insoluble products.
To know more about Double-displacement reactions, refer to the link below:
https://brainly.com/question/29307794#
#SPJ11
The solubility of KCl at 50° C is approximately 43.0 grams per 100 grams of water. When 50 grams of potassium chloride, KCl, is completely dissolved in 100 grams of water at 50 degrees Celsius, the solution can be correctly described as:
a. saturated
b. unsaturated
c. supersaturated
The solubility of KCl at 50° is approximately 43.0 grams per 100 grams of water.
Answers
If the solubility of KCl at 50°C is approximately 43.0 grams per 100 grams of water, and 50 grams of KCl is completely dissolved in 100 grams of water at 50°C, then the solution is saturated. Option A is correct.
A saturated solution is one in which the maximum amount of solute (in this case, KCl) has been dissolved in the solvent (in this case, water) at a given temperature, and no more solute can be dissolved. Since the amount of KCl that has been dissolved (50 grams) is equal to the maximum amount that can be dissolved in 100 grams of water at 50°C (43 grams), the solution is saturated.
If the amount of KCl dissolved was less than 43 grams, the solution would be unsaturated, meaning that more solute could be dissolved. If the amount of KCl dissolved was more than 43 grams (without any additional heat or other changes to the system), the solution would be supersaturated, meaning that the excess solute would start to precipitate out of solution.
Hence, A. saturated is the correct option.
To know more about saturated solution here
https://brainly.com/question/1851822
#SPJ4
Is starch an ionic or covalent compound?
Answers
Starch is a covalent compound as it possesses long chains of glucose molecules that are bonded through covalent bonds.
What are covalent compounds?In a covalent compound, the covalent bonds are involved where the mutual sharing of electrons that can hold all atoms or molecules together. Shared electrons are difficult to give away as nuclei of two atoms together share the electrons and create the bond stronger.
Ionic compounds can be demonstrated as compounds prepared between a cation and an anion. A cation can be defined as an electropositive ion and can lose valence electrons. Similarly, anions are electronegative ions and can a tendency to gain electrons.
Starch can be defined as a polysaccharide composed of 1,4 linkages between glucose monomers. The linear polymer amylose can be defined as the most basic form of starch, while amylopectin can be defined as the branched form.
Therefore, Starch is a covalent compound because of the covalent bonds between the glucose molcules.
Learn more about the covalent compound, here:
brainly.com/question/12144907
#SPJ4
Given: A 3B -- > 2C D This reaction is first order with respect to reactant A and second order with respect to reactant B. If the concentration of A is doubled and the concentration of B is halved, the rate of the reaction would _______ by a factor of ______.
Answers
In the reaction, A+ 3B -- > 2C + D the reaction is first order with respect to reactant A and second order with respect to reactant B. If the concentration of A is doubled and the concentration of B is halved, the rate of the reaction would be zero order by a factor of 1/2
The reaction given in the question is -
2A + 3 B ----> 2C + D
From , the above reaction the rate law is written as -
rate = k [ A ]²[ B ]³
where ,
k = rate constant
In the above equation the order is determined by the sum of the powers of the concentrations , i.e. 2 + 3 = 5 order , which never possible ,
Hence , in the question ,
The by changing the concentration of B , the rate does not change .
hence , the rate is independent of the concentration of B .
Therefore ,
The order with respect to B will be zero .
To know more about order of a reaction here
https://brainly.com/question/15204410
#SPJ4
During a titration the following data was collected. A 50.0 mL portion of H2SO4 was titrated with 0.500 M KOH. 200. mL of the base was needed to neutralize the acid. Calculate the molarity of the acid.
Answers
Titration is a method of chemical analysis that is used to determine the concentration of a reactant of unknown concentration.
Molarity(M) = (number of moles of solute) / (volume of solution in liters)Therefore,Molarity of the acid = (number of moles of acid) / (volume of acid)Note: We have to use the balanced chemical equation to find the number of moles of the solute.Molecular formula of H2SO4 is:H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l)Step-by-step explanation:The reaction is given by the following balanced equation:H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l)Number of moles of KOH used for titration can be calculated using the formula:Molarity(M) = (number of moles of solute) / (volume of solution in liters)The volume of KOH solution is 200 ml = 0.2 L.Molarity of KOH solution is 0.500 M.Number of moles of KOH used = Molarity × Volume = 0.500 × 0.2 = 0.1 molAccording to the balanced chemical equation,2 moles of KOH react with 1 mole of H2SO4.Therefore,1 mole of H2SO4 reacts with 2 moles of KOH.Moles of H2SO4 = (moles of KOH) / 2 = 0.1 / 2 = 0.05 molVolume of H2SO4 = 50 ml = 0.05 L.Number of moles of solute = 0.05 mol.Volume of the solution = 0.05 L.Molarity of the acid = (number of moles of acid) / (volume of acid)= 0.05 / 0.05 = 1.0
50.0 mL of H2SO4 requires 0.2 L of 0.500 M KOH. The molarity of the acid is 1.0 M.
Learn more about titration here:
brainly.com/question/31483031
#SPJ11
Show transcribed data
Calcium ions are important for many cellular processes including muscle contraction and signaling cascades. Which type of transport is most likely used to import Ca2+ into the cell?
O A Simple diffusion
o B Facilitated diffusion
O C Osmosis
Answers
Facilitated diffusion can be involved in the transport of calcium ions into the cell. Hence option B is right.
Calcium ions have a positive charge, and their hydrophobic nature prevents them from freely diffusing through the hydrophobic region of the phospholipid bilayer.
To overcome this barrier, calcium ions utilize specific transport proteins called calcium channels or calcium ionophores.
These transport proteins create pathways within the cell membrane that allow calcium ions to passively diffuse down their concentration gradient. Facilitated diffusion does not require the expenditure of energy by the cell.
These calcium channels or ionophores provide a selective pathway for the entry of calcium ions into the cell.
They recognize and bind to calcium ions, undergoing conformational changes that allow the ions to move across the membrane.
This process is crucial for calcium signaling and various cellular processes that rely on calcium ions.
Therefore, facilitated diffusion via calcium channels or ionophores is a mechanism by which calcium ions are imported into the cell.
Learn more about Facilitated diffusion at: https://brainly.com/question/28021053
#SPJ11
what is the mass of 7.525 x10^24 particles of sulfur difluoride SF2?
Answers
Answer:
Mass will be equal to 874.56 gram
Explanation:
Molar mass of sulfur = 32 u
Molar mass of Florine = 19 u
Therefore molar mass of \(SF_2\) \(=32+2\times 19=70u\)
So mass of \(6.023\times 10^{23}\) particle is 70 gram
Therefore mass of 1 particle
\(=\frac{70}{6.023\times 10^{23}}=11.62\times 10^{-23}g\)
Therefore mass of \(7.525\times 10^{24}\) particle is
\(7.525\times 10^{24}\times 11.62\times 10^{-23}=874.56gram\)
Iodine- 131 Is a radioactive isotope, and is often used in certain medical treatments. It has a short half life of about 8 days. It a hospital has a 1050 mg sample of it’s available, how much would be absolute after 72 days
Answers
After 72 days, a 1050 mg sample of Iodine-131 would have decayed to approximately 46.87 mg.
What is a half-life and how does it relate to the decay of radioactive isotopes?A half-life is the time it takes for half of a sample of a radioactive isotope to decay. It is a characteristic property of each isotope and can be used to predict how long it will take for a given sample to decay to a certain amount. The amount of an isotope remaining after a certain amount of time can be calculated using the half-life and the formula N = N0 * (1/2)^(t/T).
Why is Iodine-131 used in medical treatments and how does its short half-life factor into its use?Iodine-131 is used in medical treatments because it emits beta particles that can destroy cancerous cells. Its short half-life is an advantage because it allows for a higher dose of radiation to be delivered to the tumor while minimizing the amount of radiation exposure to healthy tissues. After a few weeks, the Iodine-131 decays to a negligible amount and the patient is no longer radioactive.
To know more about radioactive isotopes,visit:
https://brainly.com/question/1907960
#SPJ1
HELP Sometimes a retired military vessel is hauled out to sea and
intentionally sunk. After some time on the seafloor, the ship begins to
form a coral reef that is home to many sea creatures. Which of the
following best describes how this action affects the environment?
A
The number of predators in the area is decreased.
B
A new habitat is created that supports several kinds of life.
с
Many harmful toxins are introduced into the environment.
D
Plants and animals have a new source of energy to live.
Answers
Answer:
probably B, a new habitat is created that supports several kinds of life.
Explanation:
What does acetic anhydride form when it reacts with water?
Answers
What does acetic anhydride form when it reacts with water?
When acetic anhydride reacts with water it forms acetic acid. In other words, The hydrolysis of acetic anhydride in presence of water forms acetic acid.*Note that:- It is an exothermic reaction.
Read the entire procedure for the Introductory Activity. In step 5, why is it necessary to make sure the stopcock is in the open position and then immediately replace the stopper and syringe assembly in the Erlenmeyer flask after adding the hydrochloric acid to the marble chips?
Answers
When diluted hydrochloricacid is given to a reactive metal, hydrogen gas is created as a result of the addition of HCl to the reaction mixture that will result in the f.
Both hydrogen ions (H +) and chloride ions (Cl -) would be added to the equilibrium mixture if hydrochloricacid were to be added. Since hydrogen ions are on the right side of the equilibrium, it will shift to the left to make up for this, increasing the concentration of reactants. Because hydrogen ions are on the right side of the equilibrium, the equilibrium will move to the left to make up for this, which will increase the concentration of reactants.
To learn more about hydrochloricacid please click on below link
https://brainly.com/question/15102013
#SPJ4
If 5. 00L of nitrogen reacts completely with hydrogen at a pressure of 3. 00atm and a temperature of 298k, how much ammonia, in gram, is produced?
Answers
If 5.00L of nitrogen completely reacts with hydrogen at a pressure of 3.00atm and a temperature of 298k,approximately 20.67 grams of ammonia will be produced.
The balanced chemical equation for the reaction between nitrogen and hydrogen to form ammonia is:
N2 + 3H2 → 2NH3
We can use the ideal gas law to calculate the number of moles of nitrogen:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant (0.0821 L atm mol K), and T is the temperature in Kelvin.
Rearranging the equation, we get:
n = PV/RT
Substituting the given values, we get:
n = (3.00 atm) (5.00 L) / (0.0821 L atm mol K) (298 K)
n = 0.607 mol
n(NH3) = 2 × n(N2) = 2 × 0.607 mol = 1.214 mol
Mass(NH3) = n(NH3) × M(NH3) = 1.214 mol × 17.03 g/mol ≈ 20.67 g
learn more about moles here:
https://brainly.com/question/28239680
#SPJ4
0.038 moles of o2 are collected after the reaction of 78.2 ml of a 4.6 m solution of h2o2. the density of the solution is 1.4 g/ml. what is the percent yield?
Answers
The percent yield of the reaction of H2O2 is found to be 21.16%
What varies the theoretical yield from the actual yield?The theoretical yield is the yield that is obtained using a balanced chemical reaction. What you actually obtain in a chemical reaction is the actual yield. The actual yield is evaluated with the theoretical yield to establish the percent yield.
2 H2O2 => 2H2O + O2
78.2 mL of 4.6 M solution of H2O2 :
=> 78.2/1000*4.8= 0.359 mole H2O2
2 moles H2O2 ---> 1 mole O2
=> 0.359 moles H2O2-----> x mole O2
x => 0.359/2= 0.1795mole O2
0.038 mole O2 was collected
Thus, percent yield = 0.038/0.1795*100=21.16%
What does "actual yield" mean?Actual Yield is the quantity of a product discovered to be created during a chemical process.
Learn more about percentage yield here:
brainly.com/question/12704041
#SPJ4
How many grams of oxygen, at standard conditions,
are needed to react with 90 grams of glucose,
(C6H12O6)?
Postpone
C6H1206+ 602 6CO₂+ 6H₂O
2
grams
SUBMIT
Answers
In ideal conditions, 90 grams of glucose require 95.92 grammes of oxygen to react.
How many grams of oxygen are needed to oxidise 180 grammes of glucose?This tells us that 1 molecule of glucose must be oxidised by 6 molecules of oxygen (O2) (C6H12O6). Hence, 1 mole of glucose requires the oxidation of 6 moles of oxygen. Hence, 180 grams of glucose require 6 x 32 = 192 grams of oxygen to be oxidised.
Applying the balanced chemical equation
C6H12O6 + 6O2 -> 6CO2 + 6H2O
First, we need to calculate the number of moles of glucose in 90 grams:
Number of moles of glucose = mass / molar mass
Number of moles of glucose = 90 g / 180.16 g/mol (molar mass of C6H12O6)
Number of moles of glucose = 0.4996 mol
Then, we may determine how many moles of oxygen are needed by using the mole ratio between glucose and oxygen:
Number of moles of oxygen = 6 x number of moles of glucose
Number of moles of oxygen = 6 x 0.4996 mol
Number of moles of oxygen = 2.9976 mol
Lastly, we may convert the amount of moles to grammes using the molar mass of oxygen:
Mass of oxygen = number of moles x molar mass
Mass of oxygen = 2.9976 mol x 32.00 g/mol (molar mass of O2)
Mass of oxygen = 95.92 g
To know more about glucose visit:-
https://brainly.com/question/30548064
#SPJ1
What is cholesterol and how does it affect our body?
Answers
Answer:
Cholesterol is a waxy substance found in your blood. Your body needs cholesterol to build healthy cells, but high levels of cholesterol can increase your risk of heart disease. With high cholesterol, you can develop fatty deposits in your blood vessels.
Explanation:
Answer:
Cholesterol is a waxy substance found in your blood. It can affect our bodies in many ways. If you have high levels of cholesterol it can increase your risk of heart disease. Your body needs it to help build healthy cells.
Explanation:
Hope this helps:)...if not then sorry for wasting your time and may God bless you:)
specific heat (l) (j/goc) specific heat (s) (j/goc) heat of vaporization (kj/mol) heat of fusion (kj/mol) molar mass (g/mol) boiling point melting point hypothetical material 4.1 6.7 6.7 24.1 68.1 126.0 -16.0 how much heat needs to be added to the material to heat 93.3 grams of the material from 23.1oc to 61.8oc?
Answers
The heat need to be added to the material to heat 93.3 grams of the material from 23.1°C to 61.8°C is approximately 14,803 Joules
To calculate the heat needed to heat 93.3 grams of the hypothetical material from 23.1°C to 61.8°C, we will use the specific heat formula:
q = mcΔT
Where q represents the heat added, m is the mass of the material, c is the specific heat, and ΔT is the change in temperature.
In this case, we have:
m = 93.3 g (mass of the material)
ΔT = 61.8°C - 23.1°C = 38.7°C (temperature change)
For the specific heat (c), we need to consider whether the material is in its liquid (l) or solid (s) state during this temperature range. Since the melting point is -16.0°C and the boiling point is 126.0°C, the material is in its liquid state between 23.1°C and 61.8°C. Therefore, we use the specific heat for the liquid state, which is 4.1 J/g°C.
Now we can plug these values into the specific heat formula:
q = (93.3 g) × (4.1 J/g°C) × (38.7°C)
q ≈ 14803 J
Approximately 14,803 Joules of heat need to be added to the material to heat 93.3 grams of the material from 23.1°C to 61.8°C.
Know more about melting point here:
https://brainly.com/question/30148142
#SPJ11
36.0 g of Be contains how many moles?
Answers
Answer:
Explanation:
4