You will be given 4.6x10^3 mg of Sodium Chloride, how many atoms of NaCl do I have?
Answers
The total number of molecules that are present in 4.6×\(10^{3}\) mg of NaCl is 0.47×\(10^{23}\)
Atomic masses:
Na=23 , Cl=35.5
The molar mass of NaCl=58.5
1gm of NaCl contains=1/58.5 moles of NaCl
And,
1gm of NaCl contains=1/58.5×6.022×\(10^{23}\) molecules of NaCl
4.6x\(10^{3}\) mg =(4.6x\(10^{3}\)x\(10^{-3}\) gm) of NaCl contains= \(\frac{1}{58.5}\) ×6.022×\(10^{23}\)×4.6
=0.017×27.70×\(10^{23}\)
=0.47×\(10^{23}\)
Therefore the total number of molecules present in 4.6×\(10^{3}\) mg NaCl is 0.47×\(10^{23}\)
Learn more about molar mass:
https://brainly.com/question/28447857
Related Questions
The atomic masses of 35^Cl (75.53 percent) and 37^Cl (24.47 percent) are 34.968 and 36.956 amu, respectively. Calculate the average atomic mass of chlorine. The percentages in parentheses denote the relative abundances
Answers
An element can have multiple isotopes. Isotopes correspond to variations of the same element with respect to the number of neutrons in its nucleus. the number they give us, 35 and 37 correspond to the mass number of chlorine. The percentage will be how abundant the element is.
To find the average atomic mass we must multiply the mass of the isotope by its respective percentage of abundance and add these two results.
So, the average atomic mass of Cl will be:
\(AtomicMassCl=34.968amu\times75.53\%+36.956amu\times24.47\%\)\(\begin{gathered} AtomicMassCl=26.411amu+9.043amu \\ AtomicMassCl=35.454amu \end{gathered}\)Answer: the average atomic mass of chlorine is 35.454 amu
Ksp for ZnS is 1.1 x 10-21 At what s2- concentration will ZnS precipitate for a 0.20 M solution of Zn(NO3)2? Zn(NO3)2 is a very soluble salt. 1.3.3 x 10-11 M 2. 2.2 x 10-20 M 3. 5.5 10-21 M 4. 5.5 x 10-20 M 5. 2.4 x 10-10 M
Answers
The equilibrium concentration of S2−, can be x,x[ S 2 − ]=[ Zn 2 + ]=0.20 MKsp=[Zn2+][S2−]=1.1×10−21=0.20x20x=sqrt(1.1×10−21/0.20)=5.5×10−20 M[Zn2+]=[S2−]=5.5×10−20 MTherefore, the precipitating concentration of ZnS is 5.5 × 10−20 M.
Zinc sulfide is a compound that is colorless, transparent, and refractive. The mineral wurtzite is its most common form, although sphalerite occurs as a red, yellow, greenish, or black color. It is a chemical compound made up of the elements zinc and sulfur, and its chemical formula is ZnS.What is Ksp?Ksp (solubility product constant) is the equilibrium constant for a solid substance dissolving in an aqueous solution. It reflects the degree of saturation of a solution with a solute. For a compound that is ionically dissociated, it is equivalent to the product of the concentrations of the ions, each raised to the power of their stoichiometric coefficient. Zn(NO3)2 is the chemical formula for zinc nitrate. Zinc nitrate is a salt with a colorless or white crystalline appearance that is easily soluble in water and ethanol.What is the formula for Zinc sulfide?ZnS is the chemical formula for zinc sulfide.What is the formula for sulfide?The sulfide ion is a negatively charged polyatomic ion with the chemical formula S2-. It can be made by reacting an acid with a sulfide salt or by reducing sulfur with an appropriate reducing agent.ZnS will precipitate when the ion product is greater than the solubility product constant, which is equal to 1.1 x 10-21. Therefore, let's compute the equilibrium constant for the reaction ZnS(s)⇌Zn2+(aq)+S2-(aq)The equilibrium expression for this reaction isKsp=[Zn2+][S2−]The equilibrium concentration of Zn2+ can be computed from the concentration of Zn(NO3)2:0.20 M Zn(NO3)2⇌0.20 M Zn2+The equilibrium concentration of S2−, can be x,x[ S 2 − ]=[ Zn 2 + ]=0.20 MKsp=[Zn2+][S2−]=1.1×10−21=0.20x20x=sqrt(1.1×10−21/0.20)=5.5×10−20 M[Zn2+]=[S2−]=5.5×10−20 MTherefore, the precipitating concentration of ZnS is 5.5 × 10−20 M.
Learn more about equilibrium concentration here:
https://brainly.com/question/32029862
#SPJ11
Gravity is due to:
A. the pull between any objects with mass.
b. the pull between Earth and the moon.
c. the pull between atomic nuclei.
d. the pull between the Sun and Earth.
Answers
Answer:
I'm probably wrong but I wanna say C.
Explanation:
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
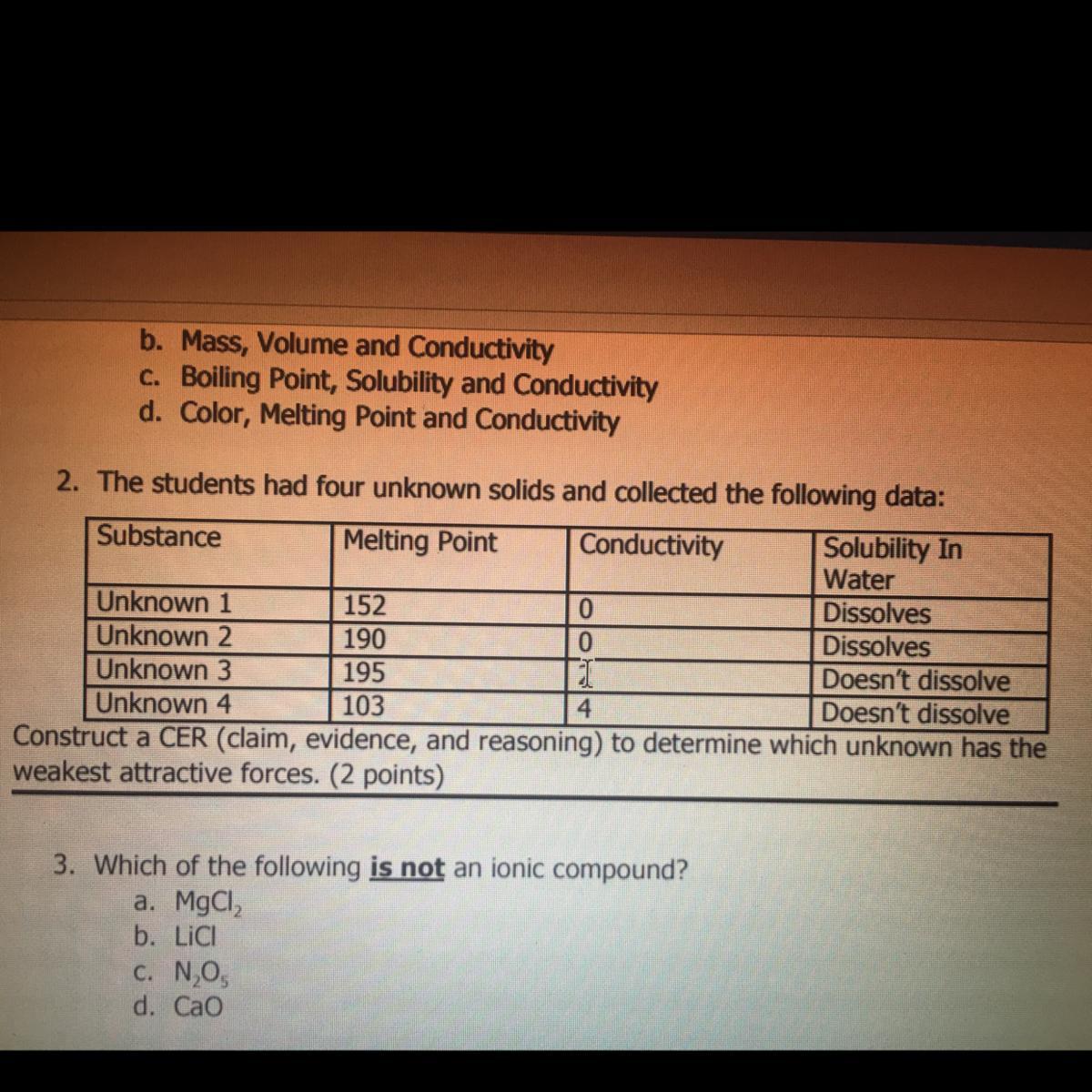
Answers
Answer:
Unknown 4
Explanation:
. Using a standard reduction table, find the cell potential of the following cell:
2 Ag+ (aq) + Sn (s) ==> Sn2+ (aq) + 2 Ag (aq)
Answers
The cell potential is 0.94 V.
To find the cell potential of the given cell, we can use the standard reduction potentials from a table.
The half-reactions are:
\(Ag^+ + e^- → Ag E° = +0.80 V\)
\(Sn^2^+ + 2e^- → Sn E° = -0.14 V\)
The overall cell potential (Ecell) can be calculated as sown below.
Ecell = E°cathode - E°anode
Ecell = (+0.80 V) - (-0.14 V)
Ecell = +0.94 V
Therefore, the cell potential of the given cell is +0.94 V.
To learn about cell potential:
https://brainly.com/question/28455231
#SPJ4
what is the mass of 1.20 x 10^25 atoms of He
Answers
Answer:
79.7 g He
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:
Step 1: Define
1.20 × 10²⁵ atoms He
Step 2: Identify Conversions
Avogadro's Number
Molar Mass of He - 4.00 g/mol
Step 3: Convert
\(1.20 \cdot 10^{25} \ atoms \ He(\frac{1 \ mol \ He}{6.022 \cdot 10^{23} \ atoms \ He} )(\frac{4.00 \ g \ He}{1 \ mol \ He} )\) = 79.7077 g He
Step 4: Check
We are given 3 sig figs. Follow sig fig rules and round.
79.7077 g He ≈ 79.7 g He
How many grams of na2o are required to produce 160 grams of NaOH? Show your work
Na2O+H2O->2NaOH
Answers
Answer:
124 grams of Na2O are required to produce 160 grams of NaOH.
Explanation:
The balanced chemical equation is: Na2O + H2O -> 2NaOH
From the equation, we can see that 1 mole of Na2O produces 2 moles of NaOH.
To find the amount of Na2O required to produce 160 grams of NaOH, we need to first calculate the number of moles of NaOH:
Molar mass of NaOH = 23 + 16 + 1 = 40 g/mol
Number of moles of NaOH = mass / molar mass = 160 g / 40 g/mol = 4 moles
Since 1 mole of Na2O produces 2 moles of NaOH, we need half as many moles of Na2O, which is 2 moles.
Finally, we can calculate the mass of Na2O required:
Molar mass of Na2O = 23 + 23 + 16 = 62 g/mol
Mass of Na2O = number of moles * molar mass = 2 moles * 62 g/mol = 124 g
Therefore, 124 grams of Na2O are required to produce 160 grams of NaOH.
Please help!!
How many moles of sodium carbonate (Na2CO3) are required to precipitate the calcium ion from 803.1 mL of a 0.35 M CaCl2 solution?
Answers
Answer:
\(n_{Na_2CO_3}=0.28molNa_2CO_3\)
Explanation:
Hello,
In this case, the undergoing chemical reaction is:
\(Na_2CO_3(aq)+CaCl_2(aq)\rightarrow CaCO_3(aq)+2NaCl(aq)\)
Hence, given the solution of calcium chloride, we can compute its reacting moles:
\(n_{CaCl_2}=0.35\frac{mol}{L}*803.1mL*\frac{1L}{1000mL}= 0.28molCaCl_2\)
Thus, by knowing there is a 1:1 molar ratio between sodium carbonate and calcium chloride, we can easily compute the moles of sodium carbonate needed for a complete precipitation as shown below:
\(n_{Na_2CO_3}=0.28molCaCl_2*\frac{1molNa_2CO_3}{1molCaCl_2} \\\\n_{Na_2CO_3}=0.28molNa_2CO_3\)
Best regards.
during prophase a group of fibers called what forms the cell
Answers
Answer:
During prophase, the nucleus disappears, spindle fibers form, and DNA condenses into chromosomes ( sister chromatids ). During metaphase, the sister chromatids align along the equator of the cell by attaching their centromeres to the spindle fibers.
Explanation:
If I beat it, I ain't wearin' a johnny (Hah)
Adeola wanna roll with a geezer (With a geez)
Is it me or the lifestyle, sweetheart?
Actually, I don't give a shi (Nah)
I'm a rapper now, might as well live in it (Live in it)
What Classification type star this?

Answers
Explanation:
The Sun is a as a G2V type star, a yellow dwarf and a main sequence star. Stars are classified by their spectra (the elements that they absorb) and their temperature.
whish this helped!
If a neutral compound is composed of carbon and hydrogen and you know that it has exactly 2 carbons connected by a double bond, how many hydrogens will the compound have?
Answers
Answer: 4 hydrogens
Explanation:
This is what the structure will look like C=C. Remember that it's important that all structures have a complete octet. As it looks right now each carbon is sharing 4 valence electrons so each needs 2 more bonds to hydrogen complete its octet.
Answer: 4 hydrogens
Explanation:
Which picture below represents the structure of a covalent compound
|
||
|||
|V

Answers
The picture that represents the image of a covalent compound is II.
What is a covalent compound?A covalent compound is a type of chemical molecule created when two or more nonmetal atoms share electrons. Atoms share electrons in covalent bonds to fill up their valence shells and increase their stability.
As a result, molecules are created, which, depending on the type of covalent bond, can be gases, liquids, or solids at room temperature. We can see that the image in II involves the sharing of electrons thus the compound is covalent.
Learn more about a covalent compound:https://brainly.com/question/30420584
#SPJ1
How does the presence of kelp influence water carbon dioxide levels?
Answers
Kelp, as photosynthesizes, remove carbon dioxide from the water, perhaps making conditions less acidic and hence more conducive for a variety of marine creatures.
Because kelp biomass is concentrated towards the surface in the canopy zone, increased kelp photosynthesis can raise dissolved oxygen and pH in surface waters relative to deeper water (Frieder et al., 2012; Koweek et al., 2017).
Kelp takes CO2 into its tissues through the process of photosynthesis. They have an exceptional potential to store 200 million tons of CO2 every year because to their fast development. However, their natural carbon sequestration capacity has been estimated to reach 1-10 billion tons per year!
Learn more about carbon dioxide
https://brainly.com/question/29968202
#SPJ4
What is the outcome of ketosis? glycogen buildup metabolic alkalosis water retention and edema glucogenesis
Answers
Metabolic acidosis would be the result of ketosis. A buildup of ketones in the body that is too great leads to metabolic acidosis.
An excess of acids in the blood can lead to metabolic acidosis. When our kidneys find it difficult to sufficiently eliminate acid from human blood, this occurs.
Because there is an excessive amount of ketone bodies in the blood, ketoacidosis would be a metabolic acidosis with a significant anion gap (keto-anions). The liver releases ketone bodies (acetoacetate, beta-hydroxybutyrate, and acetone) into the blood when the state of the liver's lipid metabolism shifts to one of greater ketogenesis.
In addition to assisting with fat loss, ketosis can reduce your appetite. It also aids in maintaining muscle. Ketosis typically begins to occur in healthy individuals who do not have diabetes and are not pregnant after 3 to 4 days of consuming just under 50 grams of carbohydrates each day.
Therefore, the outcome of ketosis will be Metabolic acidosis
To know more about ketosis.
https://brainly.com/question/28145679
#SPJ4
what’s the answer to this?

Answers
What is an isotope of the same element?
Answers
Answer:
please mark as brainliest
Explanation:
Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons and electrons . The difference in the number of neutrons between the various isotopes of an element means that the various isotopes have different masses.
example:chlorine is an example of an isotope it has a proton number of 17 and a mass number of 35 in some cases they have a proton number of 17 and a mass number of 37 there is difference in the number of neutron to calculate this we do it this way.for the first one
mass number=proton+neutron
neutron=mass number-proton
neutron=35-17
neutron=18
for the second one
neutron=37-17
neutron=20
How many of the following are WEAK acids?
HNO2 HF HNO3 H2PO4^-
a. 0
b. 1
c. 4
d. 2
e. 3
Answers
The weak acids are HNO₂ and HF. Option D is correct.
HNO₂ (nitrous acid) and HF (hydrofluoric acid) are considered weak acids because they only partially dissociate in water, resulting in a relatively low concentration of H⁺ ions in solution. On the other hand, HNO₃ (nitric acid) and H₂PO₄⁻ (dihydrogen phosphate) are strong acids, which fully dissociate in water, producing a high concentration of H⁺ ions.
On the other hand, HNO₃ (nitric acid) and H₂PO₄⁻ (dihydrogen phosphate) are both strong acids;
HNO₃ is a strong acid that fully dissociates in water, resulting in a high concentration of H⁺ ions.
H₂PO₄⁻ is a weak acid in its conjugate acid form (dihydrogen phosphate), but as H₂PO₄⁻, it acts as a weak base rather than a weak acid.
Hence, D. is the correct option.
To know more about weak acids here
https://brainly.com/question/32730049
#SPJ4
Water vapor is a greenhouse gas and is produced by burning fossil fuels. however, anthropogenic water vapor does not contribute significantly to global warming because water vapor:_________
Answers
Water vapor does contribute to the greenhouse effect, but it is not considered a primary greenhouse gas because it has a relatively short atmospheric lifetime compared to other gases such as carbon dioxide.
Additionally, the amount of water vapor in the atmosphere is largely controlled by temperature, meaning that as the atmosphere warms, more water vapor can evaporate and enter the atmosphere, but as it cools, water vapor can condense and return to the surface. Therefore, while anthropogenic emissions of water vapor do contribute to the overall concentration in the atmosphere, its impact on climate change is largely driven by other greenhouse gases.
Learn more about primary greenhouse,
https://brainly.com/question/8791277
#SPJ4
How did the Quantum Mechanical Model of the atom advance the Bohr's Model of the atom? Discuss how it added
to the Bohr Model and brought the Quantum Mechanical Model to be our current model of the atom.
Answers
Answer:
The Quantum Mechanical Model suggested that electrons do not move in fixed shells as the Bohr Model suggested, but rather acted as a cloud of electrons with orbitals electrons can most commonly be found in.
What type of weathering occurs when acid rain changes rocks? A. erosion
B. deposition C. chemical weathering D. physical weathering
Answers
Answer:
c...................................
Need help ASAP please

Answers
Answer:Melting can create steam, kind of like a nukeular plant exept no nukulear rods
2. Exothermic; decreases
3. Endothermic;increases
4. Exothermic; decreases
5. Endothermic; increases
Determine the percent composition of hydrogen inHlNH3 COOH
Answers
We will follow the following formula in order to calculate percentage composition of x element :
Percentage coposition of X = (Molar Mass of X) /(Molar Mass of whole molecule )* 100
1.Percentage composition of hydrogen in HI ( M.mass HI = 1 + 126. = 127g/mol)
% of H in HI = Molar Mass of H / Molar Mass of whole molecule *100
= (1.008g/mol /127.99g/mol ) *100
= 0.78 %
2. Percentage composition of hydrogen in NH3 ( M.mass NH3= 14+3 = 17 g/mol)
% of H in NH3 = M.mass of hydrogen / M.mass of NH3*100
= (1.008g/mol )/17.031g/mol *100
= 0.059*100
=5.9 %
3. Percentage composition of hydrogen in COOH
% of H in COOH = M.mass of hydrogen / M.mass of COOH *100
= 1.008g/mol /45g/mol *100
= 0.024*100
= 2.24 %
1) Which of these determines the identity of an element? *
O A) Number of electrons
B) Number of protons
C) Number of energy levels
Answers
Answer: The answer is B. The number of protons
Explanation:
Please Help :,D
43.5-g of cesium explosively reacts with water to form hydrogen gas and cesium hydroxide. How many moles of hydrogen gas were formed?
2Cs(s) + 2H2O(l) --> 2CsOH(s) + 1H2(g)
Calculate the mass of silver needed to react with chlorine to produce 42 g of silver chloride.
2Ag(s) + Cl2(g) --> 2AgCl(s)
Silver Nitrate reacts with sodium chloride to make the silver chloride and sodium nitrate. When 12.65 grams of silver nitrate is reacted. How many grams of silver chloride are formed?
AgNO3(s) + NaCl --> AgCl(s) + NaNO3(aq)
Calcium Carbonate decomposes into calcium oxide and a common gas, carbon dioxide. When 91.0 grams of calcium oxide is formed how many liters of carbon dioxide gas is also formed from this reaction. First find the number of moles of carbon dioxide gas actually formed in the reaction, then use the following conversion factor: 1 mol CO2(g) = 22.4 Liters of CO2(g)
CaCO3(s) + HEAT --> CaO(s) + CO2(g)
Answers
Answer:
See the attached images for solution.


What controls the DNA molecule?
Answers
Answer:
nucleotides
Explanation:
The four types of nitrogen bases found in nucleotides are: adenine (A), thymine (T), guanine (G) and cytosine (C). The order, or sequence, of these bases determines what biological instructions are contained in a strand of DNA. ... DNA is made of chemical building blocks called nucleotides.
hope that helped :)
What is the elements don’t bond with other elements because their outer shell is filled?
Answers
Answer:
Inert gases or noble gases
Explanation:
These gases have 8 outer shells meaning their full so they won't allow chemical reactions therefore providing an inert environment hence the name inert gas.
The reaction system CO(g) + 2 H2(g) = CH3OH(g) is at equilibrium. When H2 is added to the container, the reaction shifts to the right the partial pressure of Co does not change and the partial pressure of CH3OH decreases
Answers
The reaction system CO(g) + 2 H2(g) = CH3OH(g) is at equilibrium, meaning that the rate of the forward reaction is equal to the rate of the reverse reaction. If H2 is added to the container, the forward reaction becomes favoured and shifts to the right. This increases the amount of products (CH3OH) formed, and decreases the amount of reactants (CO and H2). As a result, the partial pressure of CO remains the same, while the partial pressure of CH3OH decreases.
This is due to Le Chatelier's Principle, which states that when a system at equilibrium is subjected to an external stress, the system will adjust to minimize the stress. In this case, the stress is the addition of H2, which is favouring the formation of products and disrupting the equilibrium. The system responds by decreasing the amount of products formed and shifting the reaction back to equilibrium.
Know more about equilibrium here:
https://brainly.com/question/3920294
#SPJ11
why does ionization energy increase across a period?
Answers
Answer: I
I
\/
Explanation: In general, ionization energy increases across a period and decreases down a group. Across a period, effective nuclear charge increases as electron shielding remains constant. ... The increased distance weakens the nuclear attraction to the outer-most electron, and is easier to remove (requires less energy).
Which statement describes the location of the molecules of a gas in a sealed container?

Answers
Answer:
the second option is the right one
Answer:
A
Explanation:
This answer makes the most sense because particles of a gas in a sealed container shoot around all over the container and can build pressure on the container.
A student calculated the number of moles of carbon dioxide to be 0.30 moles in a 200.0 g sample of a gaseous mixture. What is the percent composition of carbon dioxide? (molar mass = 44.01 g/mol)
A. 0.066%
B. 13%
C. 30%
D. 6.6%
Answers
Considering the definition of percentage by mass, the correct answer is option D. the percent composition of carbon dioxide is 6.6%
Percentage by massThe percentage by mass expresses the concentration and indicates the amount of mass of solute present in 100 grams of solution.
In other words, the percentage by mass of a component of the solution is defined as the ratio of the mass of the solute to the mass of the solution, expressed as a percentage.
The percentage by mass is calculated as the mass of the solute divided by the mass of the solution, the result of which is multiplied by 100 to give a percentage. This is:
\(percentage by mass=\frac{mass of solute}{mass of solution} x100\)
Percent composition of carbon dioxideIn this case, you know:
mass of solute= 0.30 moles × \(\frac{44.01 grams}{1 mole}\)= 13.203 grams (being 44.01 \(\frac{grams}{mole}\) the molar mass of carbon dioxide)mass of solution= 200 gramsReplacing in the definition of percentage by mass:
\(percentage by mass=\frac{13.203 grams}{200 grams} x100\)
Solving:
percentage by mass= 6.60 %
Finally, the correct answer is option D. the percent composition of carbon dioxide is 6.6%
Learn more about percentage by mass:
brainly.com/question/18646836?referrer=searchResults