Answers
Answer:
0.34 M
Explanation:
I assume that the compound is PbCl2.
One mole of PbCl2 contains one mol of Pb+2 and 2 moles of Cl-
Molarity (M)= moles (n) /Volume (V)
Moles Pb2+ = M x V = 0.17 V
Moles Cl- = moles Pb2+ x (2 moles Cl-/1 mole Pb2+) = 0.17 V x 2 = 0.34 V
M Cl- = moles Cl-/V = 0.34V/V = 0.34 M
Related Questions
Write the chemical formula for the following structure. You can't write subscripts so just
do full size subscript numbers.

Answers
Answer:
H2O2
Explanation:
By numbering the atoms we know about that it’s Hydrogen Peroxide
What is the molarity 10.0g of Cr(NO3)3 in 325 mL of solution
Answers
Answer:
Explanation:
molar mass Cr(NO3)3 = 238 g/mol
Convert 325 ml to liters: 325 mls x 1 L / 1000 mls = 0.325 L
Convert 10.0 g to moles: 10.0 g x 1 mol / 238 g = 0.0420 moles
Molarity = moles/liters = 0.0420 moles / 0.325 L = 0.129 M (3 sig. figs.)
The Solubility Product Constant for silver phosphate is 1.3x10^-20 .
The molar solubility of silver phosphate in a 0.223 M sodium phosphate solution is ?M
Answers
The Solubility Product Constant for silver phosphate is 1.3x10^-20 . The molar solubility of silver phosphate in a 0.223 M sodium phosphate solution is 77.51×10M.
A homogenous mixture of one or more solutes in a solvent is referred to as a solution. A typical illustration of a solution is the addition of sugar cubes to a cup of tea or coffee. Solubility is a quality that aids in the dissolution of sugar molecules. Thus, the ability of a substance (solute) to dissolve in a specific solvent can be defined as solubility. Any substance that is dissolved in a solvent and is either solid, liquid, or gas is referred to as a solute.
Ksp = [Ag⁺]³ [PO₄⁻]
1.3×10⁻²⁰=0.256³×s
s=77.51×10M
To know more about solubility, here:
https://brainly.com/question/28170449
#SPJ1
superficial zone chondrocytes can get compacted under physiological loading: a multiscale finite element analysis.
a. true
b. false
Answers
The statement "Superficial zone chondrocytes can get compacted under physiological loading: a multiscale finite element analysis" is true.
Superficial zone chondrocytes are the most exposed to mechanical loading and are therefore at risk of being compacted under physiological loading. A multiscale finite element analysis can be used to model and predict the mechanical behavior of cartilage under physiological loading, allowing for a better understanding of the factors that affect its mechanical properties.
This means that chondrocytes in the superficial zone of cartilage are at risk of being compacted under the physiological loading that it experiences in everyday life. A multiscale finite element analysis can be used to model and predict the mechanical behavior of cartilage under physiological loading, allowing for a better understanding of the factors that affect its mechanical properties.
Learn more about chondrocytes
https://brainly.com/question/28231704
#SPJ4
The theory of evolution states
Answers
Answer:
the theory of evolution states that all living things which exist today, and many more that are now extinct, evolved from simple life forms which first developed 3 billion years ago.
Explanation:
Which statement can best be concluded from the ideal gas law?
O The product of pressuré and volume of an ideal gas is proportional to the absolute temperature.
O All collisions between atoms or molecules
are perfectly elastic and are not the result of any attractive forces.
O The temperature, pressure, and volume of a gas are all related.
O The behavior of a gas
under real conditions does not obey the ideal gas law.
Answers
The product of pressuré and volume of an ideal gas is proportional to the absolute temperature.
What is an ideal gas?An ideal gas is a gas that is not real in nature. It is a hypothetical gas that obeys the ideal gas law.
Just like normal gas particles, the particles of an ideal gas also move randomly. However, unlike normal gas molecules, the molecules of an ideal gas do not interact with one another.
The ideal gas law states that the product of the pressure and the volume of a gas is proportional to the temperature of the gas.
Mathematically, the ideal gas equation is expressed as:
PV = nRT where n = number of mole of the gas and R = constant.
Thus, the best conclusion from the ideal gas law is the product of pressure and volume is directly proportional to the temperature.
More on ideal gases can be found here: https://brainly.com/question/12281987
#SPJ1
HELP help HELP!!!! Me ME me

Answers
Calcium carbonate is a common ingredient in antacids that reduces the discomfort associated with acidic stomach or heartburn. Stomach acid is hydrocholoric acid, HCl. What volume in milliliters (mL) of an HCl solution with a pH of 1.52 can be neutralized by 27.0 mg of CaCO3
Answers
Answer:
17.86mL of the HCl solution
Explanation:
The reaction of CaCO₃ with HCl is:
CaCO₃ + 2HCl → CaCl₂ + CO₂ + H₂O
The concentration of HCl with a pH of 1.52 is:
pH = 1.52 = -log [H⁺]
[H⁺] = 0.0302M = [HCl]
27.0mg = 0.0270g of CaCO₃ (Molar mass: 100.09g/mol) are:
0.0270g of CaCO₃ ₓ (1mol / 100.09g) = 2.70x10⁻⁴ moles of CaCO₃
Moles of HCl to react completely with these moles of CaCO₃ are:
2.70x10⁻⁴ moles of CaCO₃ ₓ (2 mol HCl / 1 mol CaCO₃) =
5.40x10⁻⁴ moles of HCl
As the concentration of HCl is 0.0302M, volume in 5.40x10⁻⁴ moles is:
5.40x10⁻⁴ moles of HCl * (1L / 0.0302mol) = 0.01786L =
17.86mL of the HCl solutionThe volume in milliliters (mL) of an HCl solution with a pH of 1.52 that can be neutralized by the given CaCO₃ is 17.87 mL
From the question,
We are to determine the volume of HCl that could be neutralized by the given CaCO₃
First, we will write the balanced chemical equation for the reaction
The balanced chemical equation for the reaction is
2HCl + CaCO₃ → CaCl₂ + CO₂ + H₂O
This means
2 moles of HCl is required to neutralize 1 mole of CaCO₃
Now, we will determine the number of moles of CaCO₃ present
Mass of CaCO₃ = 27.0 mg = 0.027 g
Using the formula
\(Number\ of\ moles = \frac{Mass}{Molar\ mass}\)
Molar mass of CaCO₃ = 100.0869 g/mol
∴ Number of moles of CaCO₃ present = \(\frac{0.027}{100.0869}\)
Number of moles of CaCO₃ present = 0.00026977 mole
Since
2 moles of HCl is required to neutralize 1 mole of CaCO₃
Then,
0.00053954 mole of HCl will be required to neutralize the 0.00026977 mole of CaCO₃
∴ 0.00053954 mole of HCl is required to neutralize the CaCO₃
Now, for the volume of HCl solution with a pH of 1.52 required
First,
We will determine the concentration of the HCl
From the given information
pH of the HCl = 1.52
Using the formula
pH = -log[H⁺]
Then,
1.52 = -log[H⁺]
∴ [H⁺] = 10^(-1.52)
[H⁺] = 0.0302 M
∴ The concentration of the HCl is 0.0302 M
Now, for the volume
Using the formula,
\(Volume = \frac{Number\ of\ moles}{Concentration}\)
∴ Volume of HCl required = \(\frac{0.00053954}{0.0302}\)
Volume of HCl required = 0.01787 L
Volume of HCl required = 17.87 mL
Hence, the volume in milliliters (mL) of an HCl solution with a pH of 1.52 that can be neutralized by the given CaCO₃ is 17.87 mL
Learn more here: https://brainly.com/question/14873607
The mineral manganosite, manganese(ll) oxide, crystallizes in the rock salt structure the face-centered structure adopted by NaCl) with a density of 5.365 g/cm'. Find the unit cell edge length of manganosite.
A. 444.5 pm
B. 352.8 pm
C. 280.0 pm
D. 368.2 pm
E. 417.9 pm
Answers
Answer:
A. 444.5 pm
Explanation:
We know that:
\(Density = \dfrac{mass \ of \ atoms \ in \ unit \ cell}{total \ volume \ of \ unit \ cell}\)
i.e.
\(\rho = \dfrac{n*M}{v_c * N_A}\)
\(\rho = \dfrac{n*M}{a^3 * N_A}\)
in a face-centered cubic crystal, the number of atoms per unit cell is (n) = 4
The molar mass of manganese (II) oxide \([Mn(11)O] = 70.93 \ g/mol\)
Density \(\rho\) is given as 5.365 g/cm³
Avogadro constant \(N_A\) = 6.023 × 10²³ atoms/mol
∴
\(\rho = \dfrac{n*M}{a^3 * N_A}\)
Making th edge length "a" the subject, we get:
\(a^3 = \dfrac{n*M}{\rho* N_A}\)
\(a^3 = \dfrac{4*70.93 \ g/mol}{5.365 \ g/cm^3 *6.023 * 10^{23} \ atoms/mol }\)
\(a^3= 8.78 \times 10^{-23} \ cm^3\)
\(a= \sqrt[3]{8.78 \times 10^{-23} \ cm^3}\)
a = 4.445 × 10⁻⁸ cm
a = 444.5 pm
The unit cell edge length of manganosite is equal to: A. 444.5 pm
Given the following data:
Density of NaCl = 5.365 \(g/cm^3\)We know that the molar mass of manganese (ll) oxide is equal to 70.93 g/mol.
Avogadro constant = \(6.02 \times 10^{23}\)
Since the rock salt is face-centered cubic crystal, the number of atoms per unit cell, n = 4
To find the unit cell edge length of manganosite:
For a crystal structure, density is given by the formula:
\(Density = \frac{Mass\; of\;atoms\;in\;a\;unit\;cell}{Total\;volume\;of\;a\;unit\;cell}\)
\(\rho = \frac{nM}{N_Aa^3}\)
Where:
n is the number of atoms per unit cell.\(N_A\) is Avogadro constant.a is the edge length.M is the mass.Making "a" the subject of formula, we have:
\(a=\sqrt[3]{\frac{nM}{\rho N_A} }\)
Substituting the parameters into the formula, we have;
\(a=\sqrt[3]{\frac{4 \times 70.93}{5.365 \times 6.02 \times 10^{23} }}\\\\a=\sqrt[3]{\frac{283.72}{3.23 \times 10^{24} }}\\\\a=\sqrt[3]{8.79 \times 10^{-23} }}\\\\a = 4.445 \times 10^{-8}\; meters\)
Unit cell edge length, a = 444.5 pm
Read more: https://brainly.com/question/18320053
Summarise what 'Electrolysis' is?
Answers
Answer:
Electrolysis is a process in which an electric current is used to drive a chemical reaction that would not otherwise occur.
When an electric current is passed through the cell, the ions in the electrolyte solution are attracted to the electrodes and undergo a chemical reaction at the surface of the electrodes.
hope this helps :)
Which element is in the same group as Lithium (Li)?
Carbon (C)
Potassium (K)
Chlorine (Cl)
Hydrogen (H)
Answers
Answer: Potassium (K)
Explanation:
When vectors are added or subtracted, the net force is called the _____.
will give brainliest to whoever gets it right
Answers
Answer:
resultant
Explanation:
Help! I’ll give brainliest if u get it right!

Answers
Answer:
That is the Atomic mass
Explanation:
The element symbol is S, Element name is sulfur, and
Atomic number is 16
Consider the reaction below:
2 CO(g) + O₂(g) ⇌ 2 CO₂(g)
If Kc is 2.24 × 10²² at 1273.0 °C, calculate Kp at the same temperature.
Answers
The Kc is 2.8 * 10^24
What is the Kp?In chemistry, Kp usually refers to the equilibrium constant of a reaction that involves gases. It is defined as the ratio of the partial pressures of the products to the partial pressures of the reactants, each raised to the power of their stoichiometric coefficients.
Kp= Kc (RT)^Δn
Thus;
Kc = Kp/(RT)^Δn
Kc = 2.24 × 10²² /(0.082 * 1546)^-1
Kc = 2.8 * 10^24
Thus the Kc of the reaction when we consider the concentration of the reactants is 2.8 * 10^24
Learn more about Kp:https://brainly.com/question/22074421
#SPJ1
An element's atomic number represents
the number of protons found in that element
the number of protons and electrons found in that element
the number of protons and neutrons found in that element
the number of electrons found in that element
Answers
the second options
the number of protons and electrons found in that element
Determine whether each chemical substance would remain the same color or turn pink in the presence of phenolphthalein.
Answers
Answer:
See the answer below
Explanation:
The complete question can be seen in the attached image.
Phenolphthalein is an indicator that is often utilized in an acid-base reaction to indicate the endpoints of such reactions due to its ability to change color from pink/colorless to colorless/pink depending on if the final solution is acidic or basic.
Phenolphthalein is usually colorless in acidic solutions and appears pink in basic solutions. The more basic or alkaline a solution is, the stronger the pink color of phenolphthalein. Hence;
1. Ammonia with a pH of 11 is basic, phenolphthalein will turn pink.
2. Battery acid with a pH of 1 is acidic, it will remain colorless.
3. Lime juice with a pH of 2 is acidic, it will remain colorless.
4. Mashed avocado with a pH of 6.5 is acidic, it will remain colorless.
5. Seawater with a pH of 8.5 is basic, it will turn pink.
6. Tap water with a pH of 7 is neutral, it will remain colorless

Phenolphthalein is a chemical compound with the formula\(C_{20}H_{14}O_4\). Phenolphthalein is often used as an indicator in acid-base titrations. For this application, it turns colorless in acidic solutions and pink in basic solutions
Phenolphthalein works as in:-
Colorless in acidPink in baseAccording to the question, There are 5 solutions having different ph and the indication only turns basic solution to pink
The indicator only turn the basic solution pink and these solutions are as follows,
AmmoniaSea waterTap water.Hence, these are the answer.
For more information, refer to the link:-
What are the component of black powder and why are they needed to cause an explosion
Answers
Hi There!
Black powder is an explosive powder consisting of saltpeter, sulfur, and charcoal, used chiefly in old guns fired for sport, in fireworks, and for spotting charges in practice bombs; black gunpowder.
In addition to being easily ignited by friction and impact, black powder is also extremely sensitive to flame and spark. It ignites violently when unconstrained and explodes when lit in even the slightest amount of confinement.
Thank you,
Eddie
KCIO3 -> KCI + 02
How many moles of KCI are produced if 6743 grams of KCIO3 decomposes?
Answers
55.03 moles of KCI are produced when 6743 grams of \(KClO_{3}\) decomposes
To determine the number of moles of KCl produced when 6743 grams of \(KClO_{3}\) decomposes, we need to use the concept of molar mass and the balanced chemical equation.
First, let's calculate the molar mass of \(KClO_{3}\)
The molar mass of potassium (K) is approximately 39.10 g/mol.
The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
The molar mass of oxygen (O) is approximately 16.00 g/mol.
So, the molar mass of \(KClO_{3}\) is:
(39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol) = 122.55 g/mol.
Now, we need to calculate the number of moles of \(KClO_{3}\):
Number of moles = Mass / Molar mass
Number of moles = 6743 g / 122.55 g/mol = 55.03 mol.
According to the balanced chemical equation:
2\(KClO_{3}\) -> 2 KCl + 3 O2,
we can see that for every 2 moles of \(KClO_{3}\), we obtain 2 moles of KCl.
Therefore, the number of moles of KCl produced will be equal to the number of moles of \(KClO_{3}\) since the ratio is 1:1. Thus, 55.03 moles of KCl will be produced.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
2.00 cm x 8.00 cm x 16.0 cm=
Answers
256 milliliters
FILL IN THE BLANK:
The rate of a reaction is measured by how fast a (Product Or Reactant)
is used up or how fast a
(Reactant Or Product) is formed?
Answers
Answer:
the rate of a reaction is measured by how fast a REACTANT is used up or how fast a PRODUCT is formed
if two substance are at the same temperature, their enthalpy
Answers
Answer:
cannot be measure
Hope this helps :) !!!
4. Round off the following results to three significant figures:
a) 23.01 g
Answers
the answer should be a the the question
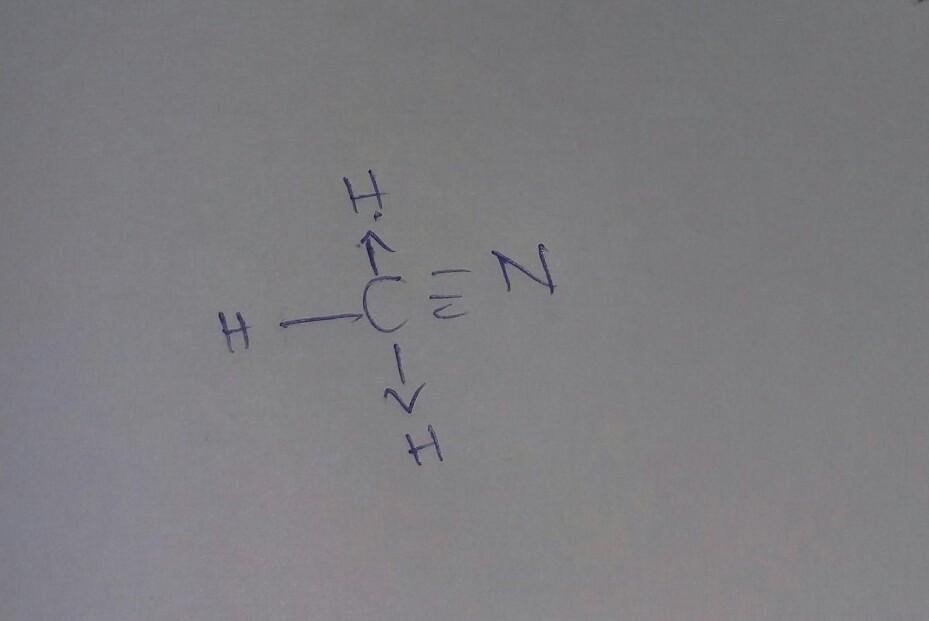
Draw a Lewis structure for the following molecules on scratch paper. Then give the number of bonds between the C and N in each.
CH5N
CH3N
HCN
Answers
Refer to the attachments


If a gas has a molecular mass of 44.0, the volume of 88.0 grams of the gas at STP would be ..
1) 44.8 L
2) 11.2 L
3) 22.0 L
4) 88.0 L

Answers
All you have to do is multiply 44 by 22.4 L, which equals 985.6
Then you divide 985.6 by 88 to get your answer of 11.2 L
HELP ME PLEASE !!!
One time I went to the mountains. I was scared of altitude sickness, so I got cannisters of oxgen (O₂). These cannisters contain 2L of compressed oxygen (O₂). When the oxygen is pressurized, it condenses into its liquid form inside the cannister. How much would this oxygen (0₂) weigh in its liquid form?
2.9 grams
1.6 grams
3.6 grams
4.3 grams
Answers
Answer:2.9 grams
Explanation:i had this question
Which statement best describes the flow of energy and the movement of
chemical compounds in an ecosystem? *
1 point
A. Energy flows into living organisms and remains there, while chemical compounds
are transferred from organism to organism.
B. Chemical compounds flow in one direction in a food chain and energy is produced.
C. Energy is transferred from organism to organism in a food chain and chemical
compounds are recycled.
D. Energy flows out of living organisms and is lost, while chemical compounds remain
permanently inside organisms.
Answers
Answer:
the answer is c
Energy is transferred from organisms in a food chain and chemical compounds are recycled
why does brain be liek fmrknve; n yyy eekf pls call for help
Answers
Answer:
man my brain like that to
Explanation:
Answer:
man my brain like that to
Explanation:
Female African elephants live in family groups that can include many adult members. Large predators will often try to isolate one
elephant from the rest of the herd because it is easier for the predator to fight and kill a single elephant
When offspring are young, they typically travel in the middle of a herd, positioned among multiple adult elephants.
Which of the following is most likely the result of adult elephants positioning their young in the middle of the herd?
A. This behavior attracts the attention of predators from far away.
B. This behavior helps adult elephants protect their offspring from predators.
C. This behavior helps adult elephants protect themselves from predators,
D
This behavior ensures that younger elephants are killed first by predators.
Answers
Answer:
B- This behavior helps adult elephants protect their offspring from predators
Explanation:
Answer: Which of the following is most likely the result of adult elephants positioning their young in the middle of the herd?
B. This behavior helps adult elephants protect their offspring from predators
Explanation:
It is because adult elephants are well aware of the dangers predators pose and want to keep their young safe from them by placing them in the center of the herd. The positioning of young elephants in the middle of the herd acts as a protective barrier, and makes it more challenging for predators to approach and attack the young elephants.The herd provides a layer of defense against potential predators, making it harder for them to isolate one elephant. This protective behavior ensures that the young elephants are less vulnerable to attacks by predators.
Which statement about balanced chemical equations is true?
OA. The mass of the new atoms that are formed equals the mass of
the atoms that made up the reactants.
OB. The total mass of the reactants equals the total mass of the
products.
OC. The total number of moles of products equals the total number of
moles of reactants,
OD. The mass of the products is greater than the mass of the
reactants when the number of moles increases.
SUBMIT
Answers
The total mass of the reactants equals the total mass of the products the statement about balanced chemical equations is true. Hence, option B is correct.
This is known as the Law of Conservation of Mass, which states that matter can neither be created nor destroyed in a chemical reaction. In other words, the mass of the reactants must equal the mass of the products in a balanced chemical equation.
While the identities of the atoms may change during a reaction, the total number of atoms of each element on both sides of the equation must be the same, thus leading to the conservation of mass.
To learn more about the balanced chemical equation, follow the link:
https://brainly.com/question/28294176
#SPJ1
why is it important to record all accidents and breakages that occur in the laboratory
Answers
It is important to record all accidents and breakages that occur in the laboratory to prevent them from happening again.
What is laboratory accident?Laboratory accident is defined as the type of accident that occurs in the laboratory leading to harm.
Example of laboratory accidents include the following:
chemical burns, cuts from broken glass, inhalation of toxic fumes, absorption of chemicals through the skin, and ingestion of toxic chemicals.A record of these type of laboratory accidents would hel prevent it's reoccurrence.
Learn more about laboratory here:
https://brainly.com/question/26264740
#SPJ1