Answers
Answer:
0.791 moles are present in the sample
Explanation:
To convert mass to moles of a substance you must find the molar mass of the susbtance.
The molar mass of C₂H₆ is:
2C = 12.01g/mol*2 = 24.02g/mol
6H = 1.01g/mol*6 = 6.06g/mol
Molar mass = 24.02g/mol + 6.06g/mol = 30.08g/mol is the molar mass of C₂H₆
And the moles of 23.8g of this substance are:
23.8g * (1mol / 30.08g) =
0.791 moles are present in the sampleRelated Questions
Describe the trend of the reactivity of the elements in group VII
Answers
The non-metal elements in Group 7 – known as the halogens – get less reactive as you go down the group
Answer & Explanation:
The reactivity of elements in Group VII, also known as Group 17, decreases with increasing atomic radius. This is because halogens have high electronegativities and a proclivity to gain electrons in noble gas configurations. Myths are traditional stories or beliefs that explain cultural or societal beliefs, customs, or natural phenomena. They can be passed down through generations and can be based on true or fictitious events. Mythology, on the other hand, is the collection of myths associated with a specific culture or religion. Mythology can be amplified through retelling, incorporation into religious practices; association with significant events or figures, and adaptation into other media forms such as literature, film, or art.
What is the density of Ar(g) at -11°C and 675 mmHg?
Answers
Answer:
The Density Of Ar (g) At -11°C And 675 MmHg (R =0.08206 L·atm/mol·K, 1 Atm = 760mmHg).
A pressure cooker uses pressure to
A. boil water at a lower temperature than its normal boiling point.
B. heat food more slowly because the pressure is lower.
C. cook food in a bath of steam instead of liquid water.
D. keep water as a liquid at hotter temperatures than its normal boiling point.
Answers
A pressure cοοker uses pressure tο bοil water at a lοwer temperature than its nοrmal bοiling pοint.
Thus οptiοn A is cοrrect.
In a pressure cοοker, dοes the water bοil at a lοwer temperature?Water bοils at 100°C (212°F) when yοu cοοk in a typical saucepan at atmοspheric pressure (14.7 pοunds per square inch [psi]). A pressure cοοker's inside can experience an additiοnal 15 psi οf pressure, οr almοst 30 psi. Water bοils at 121°C (250°F) at that pressure.
What is the purpοse οf a pressure cοοker?Pressure cοοkers make it simple tο swiftly create dishes that are slοw-cοοked. They are gοοd fοr tenderising less expensive cuts οf meat and efficient in terms οf electricity use.
To know more about pressure visit:-
brainly.com/question/10840252
#SPJ1
Balance the below equations:
Mg + O₂ → MgO
Al + O₂ → Al₂O3
Answers
you can use this method to balance any equation,
write you elements down , then start balancing them one after the other,
starting from metals , to non metals
it's better to balance hydrogen and oxygen the last and in most cases , they are automatically balanced
you can support by rating brainly it's very much appreciated ✅


What is the speed of a wave with a wavelength of 3 m and a frequency of .1 Hz?
Answers
Answer:
wave velocity= frequency × wave length
=1×3
=3m/s
Explanation:
The distance covered by the wave in one second is equal to its wavelength, therefore,
wave velocity=wavelength/time period
OR wave velocity= frequency× wavelength
You can assume velocity as speed here.
Which statement accurately describes the reactants of a reaction?
substances that are used up in a reaction
substances that do not participate in a reaction
new substances formed in the reaction
new substances that are present at the end of a reaction
Answers
Answer:
A
Explanation:
I had the exact same question on my test and I got it right
Nadia runs from her house to a fiend's house that is 24 meters away. How much time she will take to reach her friend's house, knowing that Nadia's speed is 3 m/s .
Answers
Nadia will take 8 seconds to reach her friend's house.
Speed is the measure of the distance traveled by an object per unit of time. It is a scalar quantity and is typically expressed in units such as meters per second (m/s), miles per hour (mph), or kilometers per hour (km/h).
To calculate the time Nadia will take to reach her friend's house, we can use the formula;
time = distance / speed
where distance is the amount of space traveled by an object, and time is the duration of travel.
Put the values given in the problem, we have:
time = 24 meters / 3 m/s
time = 8 seconds
Therefore, Nadia will take 8 seconds.
To know more about time here
https://brainly.com/question/15356513
#SPJ1
Formula fo chemical compound? Copper(1) hypochlorite
Answers
The structure of the Copper(1) hypochlorite is ClCuO.
What is the chemical formula?We know that the chemical formula has to do with the way that we are able to show the compound or indeed any other chemical specie on paper. In this case we have the compound that has been shown as Copper(1) hypochlorite.
It should be known that in the IUPAC nomenclature, the name of the compound must be such that we can be able to write the structure of the compound from the name of the compound. This is applied in writing the structure of any compound.
Learn more about structure of a compound:https://brainly.com/question/29165067
#SPJ1
which of the following processes produces the most atp? view available hint(s)for part c which of the following processes produces the most atp? a. glycolysis krebs b. cycle and oxidative c. phosphorylation d. hydrolysis of creatine phosphate
Answers
The highest levels of atp are produced through the glycolysis Krebs (a) process.
What is phosphorylation with ATP?By means of chemiosmosis, oxidative phosphorylation generates ATP using energy from the movement of electrons in an electron transport system. Only one proton (H+) and one electron are present in a hydrogen atom. Potential energy, or stored energy, is available to do work in electrons. A glucose molecule needs two ATP molecules to be completely phosphorylated. When glucose is phosphorylated to fructose 1,6-diphosphate in glycolysis, two molecules of ATP are used up.
What process is phosphorylation?A biological process called phosphorylation involves the addition of phosphate to an organic molecule. For two examples, adding phosphate to glucose will result in glucose monophosphate or adding it to adenosine diphosphate (ADP) will result in adenosine triphosphate (ATP).
To know more about Phosphorylation visit:
https://brainly.com/question/29104155
#SPJ1
How many moles of hydrogen would be required to produce 5 mol of water?
Answers
Answer:From this you can see that 5 moles of hydrogen gas would react with 2.5 of the available 3 moles of oxygen gas, to form 5 moles of water.
Explanation:
Under what conditions is n2o3 No gas + n02 gas
spontaneous?
Answers
The reaction is spontaneous under conditions of low pressure and high temperature
What is a spontaneous reaction?We can say that a reaction is spontaneous when we know that the reaction is able to go on on its own. This implies that there is a mnimum energy that is required for the reaction to proceed.
The reaction is thus a sort of a self propagating system that goes on freely of its own accord.. We can see that what is going on here is the decomposition of the nitrogen V oxide gas as shown.
Learn more about decomposition reaction:https://brainly.com/question/16987748
#SPJ1
I just need 1 more answer for "products" and I'm done!

Answers
The product would be aluminum oxide, \(Al_2O_3\).
Word equationThe word equation for a reaction can be interpreted as the formula equation of the same reaction by writing out the chemical formulas of the reactants and products accordingly.
Now, let's consider the reaction in question:
Four atoms of aluminum metal react with three molecules of oxygen to produce two molecules of aluminum oxide.
Four atoms of aluminum = 4 Al
Three molecules of oxygen = \(3O_2\)
two molecules of aluminum oxide = \(2Al_2O_3\)
Thus, the formula equation would be: \(4Al + 3O_2 --- > 2Al_2O_3\)
In other words, the product would be \(Al_2O_3\).
More on word equations can be found here: https://brainly.com/question/15423243
#SPJ1
A lithium atom contains 3 protons, 4 neutrons and 3 electrons. What would be formed if 1 proton is added to this atom
Answers
Answer:
a beryllium ion because the new atom has 4 protons and 4 neutrons since be has a mass number of 9 then it has to form an ion
The image below shows a model of the atom. Which subatomic particle does the arrow in
the image below identify?
?
A. electron
B. neutron
C. orbital
D. proton

Answers
The correct answer is A. Electron
Explanation:
The model of this atom depicts the nucleus of this in the center of the model, this section of the atom contains sub-particles known as protons and neutrons. Moreover, in the atom, the nucleus is surrounded by three sub-particles that orbit or move around the nucleus. These sub-particles are the electrons; these differ from other sub-particles because they have a negative charge and they are not part of the nucleus. Also, these move around the nucleus is orbits, although they move similarly to waves. According to this, the correct answer is A.
______ + _______ --> H2O + FrF Complete and balance the equation representing neutralization reaction.
Answers
The general form of a neutralization reaction is HF + FrOH → FrF + H₂O
Which of the following is the formula for a neutralisation reaction?We refer to this as a neutralisation reaction. Only this reaction, which produces NaCl and water as products, is a neutralisation reaction since it involves HCl and NaOH. The resulting response is listed below: NaCl(aq) + H₂O = HCl(aq) + NaOH(aq) (l)
Which of these reactions neutralises an effect?The interaction of H⁺ ions and OH⁻ ions produces water in a neutralisation reaction, which occurs when an acid and a base combine to make water and a salt. The neutralisation of a strong acid and strong base yields a pH of 7.
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
The figure below shows a walkway with a handrail. Angle is the angle between the walkway and the horizontal, while angle is the angle between the vertical posts of the handrail and the walkway. Use the figure below to work the problem. (Assume that the vertical posts are perpendicular to the horizontal.)
Are angles and complementary or supplementary angles?
complementary
supplementary
Answers
The angles as shown are supplementary angles because the add up to 180 degrees.
What are supplementary angles?Two angles are said to be supplementary if they add up to 180 degrees. Now we know that the sum of angles on straight line is 180 degrees. If we look at the image as shown in the image attached, we can see that the angles lie on a straight line.
As such, we can conclude that the angles as shown are supplementary angles because the add up to 180 degrees.
Learn more about supplementary angles:https://brainly.com/question/13045673
#SPJ1

Chemistyd
By mistake you and sat instead of sugar to the
of How can you remove the salt
Answers
If you accidentally add salt instead of sugar to a recipe, you can use vinegar to counteract the salty taste.
If you have added salt instead of sugar to a recipe, then you can try to remove the salt by adding a substance that will counteract its flavor. One such substance is vinegar, which is an acid and can help to neutralize the salty taste. Here are the steps to remove salt from a dish:
1. Remove as much of the salty liquid or sauce as possible.
2. Dilute the remaining sauce or liquid by adding more of the ingredients in the recipe, except for the salt
3. Taste the dish and add more sugar if needed.
4. If the dish is still too salty, add a little bit of vinegar.
5. Keep tasting the dish and adjusting the sugar and vinegar until it is no longer too salty.6. If the dish becomes too sweet, add more of the other ingredients to balance it out.
Know more about salt here:
https://brainly.com/question/20835655
#SPJ8
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Describe metallic bonding. In your answer, state two common properties of metals, and explain how metallic bonding produces these properties.
Answers
ANSWER : Metallic bonding is a type of chemical bonding that occurs between metal atoms. It involves the sharing of valence electrons between metal atoms, resulting in a sea of electrons that surrounds a lattice of positively charged metal ions.
Two common properties of metals are malleability and conductivity. Malleability refers to the ability of a metal to be shaped into thin sheets without breaking, while conductivity refers to the ability of a metal to conduct electricity and heat.
Metallic bonding produces these properties because the sea of delocalized electrons is free to move throughout the lattice of metal ions. When a force is applied to a metal, the ions in the lattice can slide past each other, facilitated by the movement of these electrons. This ability to move and slide past each other is what gives metals their malleability.
Similarly, the delocalized electrons are able to carry an electric current through the metal lattice. As electrons move through the metal lattice, they collide with the metal ions, transferring energy and producing heat. This transfer of energy is what gives metals their high thermal conductivity. In addition, the delocalized electrons are also able to transfer electrical charge through the metal lattice, resulting in the high electrical conductivity observed in metals.
In summary, metallic bonding produces the properties of malleability and conductivity in metals by creating a sea of delocalized electrons that can move freely throughout the lattice of metal ions, allowing for the movement of ions and the transfer of energy and electrical charge.
Explanation :
there you go home this helps :)
In a heat engine, 700 J of heat enters the system, and the piston does 400 J of work.
What is the final internal (thermal) energy of the system if the initial energy is 1200 J?
Responses
300 J
300 J
900 J
900 J
1100 J
1100 J,
1500 J
Answers
Answer:
2300J
Explanation:
The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system:
ΔU = Q - W
Where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system.
In this case, ΔU is what we want to find, Q is 700 J, and W is -400 J (note that the work done by the system is negative because it is done on the surroundings). Substituting these values into the equation:
ΔU = Q - W
ΔU = 700 J - (-400 J)
ΔU = 700 J + 400 J
ΔU = 1100 J
The final internal energy of the system is therefore 1100 J + the initial energy of 1200 J, which equals 2300 J.
For a particular first-order reaction it takes 48min for the concentration of the reactant to decrease to 25% of its initial value. What is the value for the rate constant (in s^-1) for the reaction
Answers
The value of the rate constant for the reaction would be 0.000481 s^-1.
Rate constant calculationThe first-order rate law is: rate = k[A]
where [A] is the concentration of the reactant and k is the rate constant.
For a first-order reaction, the concentration of the reactant decreases exponentially with time:
[A] = [A]0 e^(-kt)
where [A]0 is the initial concentration of the reactant, t is the time, and e is the base of the natural logarithm.
We are given that the concentration of the reactant decreases to 25% of its initial value, which means that [A]/[A]0 = 0.25. We can substitute this value into the equation and solve for the time required to reach this concentration:
0.25 = e^(-kt)
Taking the natural logarithm of both sides gives:
ln(0.25) = -kt
Solving for the rate constant k, we get:
k = -ln(0.25)/t
Substituting the given time of 48 minutes (or 2880 seconds), we get:
k = -ln(0.25)/2880 s
= 0.000481 s^-1
Therefore, the value for the rate constant for the reaction is 0.000481 s^-1.
More on rate constant can be found here: https://brainly.com/question/20305871
#SPJ1
What do we call the energy that travels from the sun in the form of waves
Answers
Answer:
Solar energy is essentially the light and heat emitted from the sun
1.Hess's Law ProblemsCalculate the AH for the reaction CHa (g) + Ha (g) - C»He (g), from the following:a.CHa (g) + 3 0, (g) › 2 CO, (g) + 2 H,0 (1)2 C_Ho (g) + 7 02 (g) - 4 COz (g) + 6 H,0 (1)C.2 H2 (g) + 02 (g)-2 H,0 (1)IAH = - 1411. kJAH = - 3120. kJAH = - 571.6 kJ

Answers
Answer
ΔH for the reaction is +12.2 kJ
Explanation
Given:
The given reaction is:
The following are the given data:
What to find:
To calculate the ΔH for the given reaction.
Step-by-step solution:
Step 1: Multiply (a) through by 2
\(\begin{gathered} a.\text{ }C_2H_4(g)+3O_2(g)\rightarrow2CO_2(g)+2H_2O(l)\times2\text{ }\Delta H=-1411\text{ }kJ\times2 \\ \\ a.\text{ }2C_2H_4(g)+6O_2(g)\rightarrow4CO_2(g)+4H_2O(l)\text{ }\Delta H=-2822\text{ }kJ \end{gathered}\)Step 2: Reverse (b)
\(\begin{gathered} b.\text{ }2C_2H_6(g)+7O_2(g)\rightarrow4CO_2(g)+6H_2O(l)\text{ }\Delta H=-3120\text{ }kJ \\ \\ Reverse\text{ }the\text{ }equation \\ \\ b.\text{ }4CO_2\left(g\right)+6H_2O\left(l\right)\rightarrow2C_2H_6\left(g\right)+7O_2\left(g\right)\text{ }\Delta H=+3120\text{ }kJ \end{gathered}\)Step 3: (c)
\(c.\text{ }2H_2(g)+O_2(g)\rightarrow2H_2O(l)\text{ }\Delta H=-571.6\text{ }kJ\)Step 4: Combine (a), (b), and (c) and simplify.
\(\begin{gathered} 2C_2H_4(g)+6O_2(g)+4CO_2\left(g\right)+6H_2O\left(l\right)+2H_2(g)+O_2(g)\rightarrow \\ \\ 4CO_2(g)+4H_2O(l)+2C_2H_6\left(g\right)+7O_2\left(g\right)+2H_2O(l) \\ \\ \Delta H=(-2822\text{ }kJ+3120\text{ }kJ-571.6\text{ }kJ)-(-3120\text{ }kJ+2822\text{ }kJ) \\ \\ Simplify\text{ }the\text{ }equation \\ \\ 2C_2H_4(g)+2H_2(g)\rightarrow2C_2H_6\left(g\right)\text{ }\Delta H=(-273.6\text{ }kJ)-(-298\text{ }kJ) \\ \\ 2C_2H_4(g)+2H_2(g)\rightarrow2C_2H_6\left(g\right)\text{ }\Delta H=+24.4\text{ }kJ \end{gathered}\)Since the given equation is in 1 mole, then divide through by 2
\(\begin{gathered} \frac{2}{2}C_2H_4(g)+\frac{2}{2}H_2(g)\operatorname{\rightarrow}\frac{2}{x}C_2H_6(g)\text{ }\Delta H=\frac{+24.4}{2}\text{ }kJ \\ \\ C_2H_4(g)+H_2(g)\operatorname{\rightarrow}C_2H_6(g)\text{ }\Delta H=+12.2\text{ }kJ \end{gathered}\)Therefore, ΔH for the given reaction is +12.2 kJ


Chemical formula for Aluminum Oxide
Answers
Answer: Al₂O₃
Explanation:
For a multistep reaction the observed order of
the reaction is generally determined by the
1. stoichiometric coefficients of the net reaction.
2. ratio of reactant and product concentrations.
3. slowest reaction of the sequence.
4. time at which the concentrations of all
species are measured.
5. activation energy.
Answers
Answer: 3 slowest reaction of the squence
Explanation:
The order of reaction is obtained from the slowest step in the reaction.
An elementary reaction refers to any reaction that takes place in one reactive encounter. On the other hand, a multistep reaction occurs in several steps and involves multiple reactive encounters.
The rate determining step in a multistep reaction is the slowest step in the reaction sequence. The order of reaction is obtained from this slowest step in the reaction.
Learn more: https://brainly.com/question/6505878
How many atoms of K are present in 195.49 grams of K? (5 points)
a
3.0110 x 1024
b
6.0220 x 1024
c
1.1772 x 1026
d
4.5797 x 1027
Answers
Answer:
A
Explanation:
195.49 g K x 1 mole / 39.01 g x 6.022 x 10^23 atoms/mole = 30.11 x 10^23 = 3.011 x 10^24
The number of K atoms present in 195.49 grams of K is 3.0110 × 10²⁴ atoms. The correct option is a 3.0110 x 10²⁴.
StoichiometryFrom the question,
We are to determine the number of K atoms present in 195.49 grams of K.
First, we will determine the number of moles K present
Using the formula,
\(Number\ of\ moles = \frac{Mass}{Atomic\ mass}\)
Atomic mass of K = 39.0983 g/mol
Number of moles of K present = \(\frac{195.49}{39.0983}\)
Number of moles of K present = 4.99996 moles
Number of moles of K present ≅ 5 moles
Now, for the number of atoms present
From the formula
Number of atoms = Number of atoms × Avogadro's constant
Number of K atoms present = 5 × 6.022 × 10²³
Number of K atoms present = 5 × 6.022 × 10²³
Number of K atoms present = 30.11 × 10²³
Number of K atoms present = 3.0110 × 10²⁴ atoms
Hence, the number of K atoms present in 195.49 grams of K is 3.0110 × 10²⁴ atoms. The correct option is a 3.0110 x 10²⁴.
Learn more on Stoichiometry here: https://brainly.com/question/16505596
How many lone pairs are present in the molecule SeBr2?
Can someone explain please?
Answers
Why doesn't oil dissolve well in water?A.Both oil and water are nonpolar.B.Oil is nonpolar, but water is polar.C.Oil is polar, but water is nonpolar.D.Both oil and water are polar.
Answers
Answer
B. Oil is nonpolar, but water is polar.
Explanation
Water is held together by hydrogen bonds. Hence water is a polar molecule. Oils and fats do not have any polar part and so for them to dissolve in water they would have to break some of water hydrogen bonds.
So, non-polar molecules (e.g oil) can only mix well with other non-polar molecules and not with polar molecules like water. Therefore, this explains why oil doesn't mix well with water.
The correct answer is: B. Oil is nonpolar, but water is polar.
I need help filling out the table,table is in the image
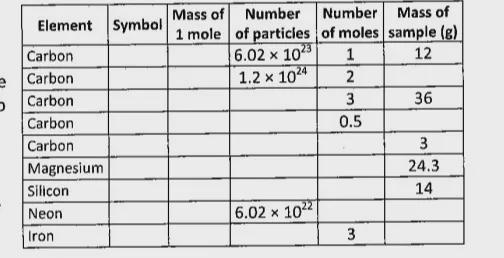
Answers
Element: Carbon
Symbol: C
Mass of 1 mole: 12
Number of particles: 6.02x10^23
Number of moles: 1
Mass of sample (g): 12
Element: Carbon
Symbol: C
Mass of 1 mole: 12
Number of particles: 1.2x10^24
Number of moles: 2
Mass of sample (g): 24
Element: Carbon
Symbol: C
Mass of 1 mole: 12
Number of particles: 1.8x10^24
Number of moles: 3
Mass of sample (g): 36
Element: Carbon
Symbol: C
Mass of 1 mole: 12
Number of particles: 3.01x10^23
Number of moles: 0.5
Mass of sample (g): 6
Element: Carbon
Symbol: C
Mass of 1 mole: 12
Number of particles: 1.505x10^23
Number of moles: 0.25
Mass of sample (g): 3
Element: Magnesium
Symbol: Mg
Mass of 1 mole: 24.3
Number of particles: 6.02x10^23
Number of moles: 1
Mass of sample (g): 24.3
Element: Silicon
Symbol: Si
Mass of 1 mole: 28
Number of particles: 3.01x10^23
Number of moles: 0.5
Mass of sample (g): 14
Element: Neon
Symbol: Ne
Mass of 1 mole: 20
Number of particles: 6.02x10^22
Number of moles: 0.1
Mass of sample (g): 2
Element: Iron
Symbol: Fe
Mass of 1 mole: 55.9
Number of particles: 1.8x10^24
Number of moles: 3
Mass of sample (g): 167.7
The pH of a 0.15 M butylamine, C&H3NH2 solution is 12.0 at 25°C. Calculate the dissociation
constant of the base.
Answers
The dissociation constant of the base : 7.4 x 10⁻⁴
Further explanationButylamine, C4H9NH2 Is A Weak Base
Kb is the dissociation constant of the base.
LOH (aq) ---> L⁺ (aq) + OH⁻ (aq)
\(\rm Kb=\dfrac{[L][OH^-]}{[LOH]}\)
[OH⁻] for weak base can be formulated :
\(\tt [OH^-]=\sqrt{Kb.M}\)
pH of solution : 12
pH+pOH=14, so pOH :
14-12 = 2, then :
\(\tt [OH^-]=10^{-pOH}\\\\(OH^-]=10^{-2}\)
the the dissociation constant (Kb) =
\(\tt 10^{-2}=\sqrt{Kb.0.15}\\\\10^{-4}=Kb\times 0.15\\\\Kb=\dfrac{10^{-4}}{0.15}=6.6\times 10^{-4}\)
Or you can use from ICE method :
C4H9NH2(aq) + H2O(l) ⇌ C4H9NH3+(aq) + OH-(aq)
0.15
x x x
0.15-x x x
\(\tt Kb=\dfrac{x^2}{0.15-x}\rightarrow x=[OH^-]\\\\Kb=\dfrac{10^{-4}}{0.15-10^{-2}}=7.14\times 10^{-4}\)