You have 40 mL of 0.10M solution of HClO with a Ka of 3.0 x 10-8. What is the pH of the solution after adding 20 mL of 0.20M NaOH?
Answers
The pH of the solution after adding 20 mL of 0.20M NaOH can be calculated as follows; Write the balanced equation for the reaction between HCIO and NaOHHCIO + NaOH → NaCIO + H2OStep 2: Determine the number of moles of HCIO initially present in the solution.Number of moles of HCIO = concentration × volume = 0.1 mol/L × 0.04 L = 0.004 mo
Determine the number of moles of NaOH added.Number of moles of NaOH = concentration × volume = 0.2 mol/L × 0.02 L = 0.004 mol
Step 4: Determine the limiting reagent.The limiting reagent is HCIO as it will react completely with the number of moles of NaOH added.'
Step 5: Determine the number of moles of HCIO that reacted with NaOH.Number of moles of HCIO that reacted = 0.004 mol
Step 6: Determine the number of moles of HCIO that remained.Number of moles of HCIO that remained = 0.004 mol - 0.004 mol = 0 mol
Step 7: Determine the concentration of HCIO after the reaction.CHO concentration = remaining volume = 40 mL + 20 mL = 60 mL = 0.06 LCHO concentration = 0 mol/L = 0/0.06 LCHO concentration = 0 mol/L
Step 8: Determine the concentration of CIO-.CIO- concentration = concentration of NaOH added = 0.2 mol/L.
Step 9: Determine the concentration of H+.H+ concentration = Ka × (CHO / CIO-)H+ concentration = (3.0 × 10-8) × (0 / 0.2) = 0 pH solution = -log[H+]pH solution = -log(0) = infinity
Therefore, the pH of the solution after adding 20 mL of 0.20M NaOH is infinity.
Know more about concentration here;
https://brainly.com/question/10725862
#SPJ11
Related Questions
please help asap in 10 mins
What are the conditions necessary for electro-chemical corrosion to occur?
Answers
Answer:
Presence of an Electrolyte
Metal Surface
Oxygen or Other Oxidizing Agent
Difference in Potential
Electrochemical Pathway
Explanation:
HELP ASAP!!!
WAVES ALWAYS ORIGINATE WITH SOME
a
person
b
object
C
disturbance
Answers
Answer:
Disturbance
Explanation:
I believe it is Disturbance because waves are normally caused by wind, and wind classifies as Disturbance.
Which of the following statements is true about osmoses? A. Osmosis will always cause crenation in cells.B. Only water and small solute particles travel through the semipermeable membrane.C.Only solute particles travel through the semipermeable membrane.D.Water will flow from high solute concentration to low solute concentration.E.none are correct
Answers
Osmosis is the movement of water through a semipermeable membrane.
From the given choices none of them is completely true.
Osmosis not always causes crenation.
In osmosis, solute particles do not travel through the membrane.
And water flows from a low solute concentration to a high solute concentration.
It means that the correct answer is E. none are correct.
A set of solubility data is given below.
What is the mass of the dry solute
recovered?
Sample
2
Temperature
(°C)
30.1
Boat Mass
(8)
0.730
Boat +
Solution (g)
0.929
Boat + Dry
(g)
0.816

Answers
Answer:
0.086
Explanation:
got it on acellus
The mass of the dry solute recovered from the given data is 0.086 g. Option C
To determine the mass of the dry solute recovered, we need to subtract the mass of the boat from the mass of the boat with the dry solute.
Given the data provided:
Boat Mass: 0.730 g
Boat + Solution: 0.929 g
Boat + Dry: 0.816 g
To find the mass of the dry solute, we subtract the boat mass from the boat + dry mass:
Mass of Dry Solute = (Boat + Dry) - (Boat Mass)
Mass of Dry Solute = 0.816 g - 0.730 g
Mass of Dry Solute = 0.086 g
Therefore, the correct answer is c) 0.086 g.
The mass of the dry solute recovered from the given data is 0.086 g. It is important to note that the mass of the dry solute is obtained by subtracting the mass of the boat from the mass of the boat with the dry solute, as the boat mass represents the weight of the empty boat or container used in the experiment.
For more such questions on solute visit:
https://brainly.com/question/25326161
#SPJ8
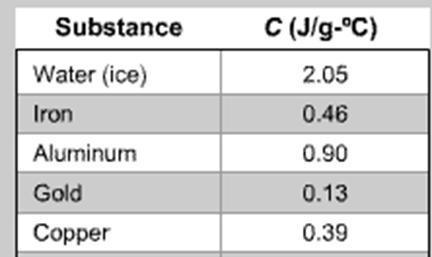
How much heat in Joules is needed for an increase in temperature by 12.5 degrees of an 8 gram block of gold
q = m • C • ΔT
C= specific heat capacity, J/(g•C)
q= heat, Joules
m= mass(grams),
ΔT= change in temperature (Celsius or Kelvin))
Question 12 options:
1.56
1000
13
100
Answers
Answer: 13
Explanation:
\(Q=mc \Delta T\\\\Q=(8)(0.13)(12.5)\\\\Q=\boxed{13}\)
A teller at a drive-up window at a bank had the following service times (in minutes) for 20 randomly selected customers: What are the 3 -sigma control limits? Select one: a. None of the other options.
Answers
Since the exact values of the service times are not provided, it is not possible to calculate the 3-sigma control limits. Therefore, the correct answer is "None of the other options."
The 3-sigma control limits are used in statistical process control to determine the acceptable range of variation in a process. To calculate the 3-sigma control limits, we need to first find the mean and standard deviation of the service times for the 20 randomly selected customers.
Step 1: Find the mean (average) of the service times.
Add up all the service times and divide by the total number of customers (20).
Step 2: Find the standard deviation of the service times.
Calculate the difference between each service time and the mean, square each difference, sum up all the squared differences, divide by the total number of customers (20), and then take the square root of the result.
Step 3: Calculate the 3-sigma control limits.
Multiply the standard deviation by 3 and add/subtract the result to/from the mean. This will give you the upper and lower control limits.
Since the exact values of the service times are not provided, it is not possible to calculate the 3-sigma control limits. Therefore, the correct answer is "None of the other options."
Learn more about 3-sigma control limits from given link: https://brainly.com/question/17063477
#SPJ11
if 30 ml of a 0.80 m solution of k is mixed with 50 ml of a 0.45 m solution of clo−4, will a precipitate be observed? the ksp for the following equilibrium is 0.004. kclo4(s)↽−−⇀k (aq) clo−4(aq)
Answers
If 30 ml of a 0.80 m solution of k is mixed with 50 ml of a 0.45 m solution of clo−4, a precipitate will be observed in this solution.
The solution contains k (potassium) and clo−4 (chlorate) ions and we are to find out if a precipitate will form or not. The ksp for the following equilibrium is 0.004. kclo4(s)↽−−⇀k (aq) clo−4(aq)
We can obtain the molarity of k ions as follows: 0.80 M = (moles of K)/(0.030 L)Moles of K = 0.80 M × 0.030 L = 0.024 mol
We can obtain the molarity of clo−4 ions as follows: 0.45 M = (moles of clo−4)/(0.050 L)Moles of clo−4 = 0.45 M × 0.050 L = 0.0225 mol
The concentration of K and clo−4 ions are 0.8 M and 0.45 M respectively. Now, we need to calculate the reaction quotient Q of the solution to find out whether the precipitate will form or not. Q = [K+][clo−4] = 0.8 M × 0.45 M = 0.36
Since Q (0.36) > Ksp (0.004), the reaction quotient is greater than the solubility product constant. It indicates that the product is more than what it should be. The excess products will precipitate to form a solid. Hence, we can say that a precipitate will be observed in this solution.
More on precipitate: https://brainly.com/question/30904755
#SPJ11
please help Why can we listen to radio or watch TV indoors?
A. It will work as long as the room has hardwood.
B. Walls absorb most of the incoming waves
C. It will work as long as the room is carpeted.
D. Walls don’t absorb the incoming waves
Answers
Answer:
D
Explanation:
A: not true. You could have cement floors and still receive radio/TV
B: not true. Very little of the waves are absorbed by the walls.
C: not true. You can receive radio/TV if there isn't a carpet in the entire house.
D: Dis the answer. Walls don't absorb the incoming waves or not much of them
If we increase the temperature of the vessel to 450 K at constant volume, what would the pressure inside the vessel be?
Group of answer choices
10 atm
5 atm
20 atm
15 atm
Answers
If we increase the temperature of the vessel to 450 K at constant volume, then the pressure inside the vessel be 15 atm.
The relationship between temperature and pressure for a fixed volume is described by Gay-law. According to this Law, the pressure of a gas is precisely proportional to its temperature for a fixed volume of the gas. It is written mathematically as P ∝ T, where P is the gas pressure.
T is the gas's temperature (in Kelvin).
P = kT, where k is the proportionality constant, and P / T is equal to k.
In this scenario, the vessel's temperature rises from 300 K to 450 K, its volume doesn't change, and its internal pressure rises.
If the temperature doubles, the pressure will also double.
To learn more about Gay-Lussac law, refer:
brainly.com/question/2644981
#SPJ4
Energy in vs Energy out = Energy balance. Explain this concept, give examples and provide support for your explanation.
Answers
The concept of energy balance refers to the equilibrium between the energy input into a system and the energy output from that system. It is based on the principle of conservation of energy, which states that energy cannot be created or destroyed but can only be transferred or transformed from one form to another.
In terms of human energy balance, it involves the energy intake from food and beverages (energy in) and the energy expenditure through basal metabolic rate, physical activity, and other bodily processes (energy out). When the energy intake matches the energy expenditure, there is an energy balance. However, when there is an imbalance, either an excess or deficit of energy, it can lead to weight gain or weight loss, respectively.
For example, if a person consumes 2000 calories (energy in) through their diet and expends 2000 calories (energy out) through their daily activities and bodily functions, they maintain an energy balance. This means that the energy intake is equal to the energy expenditure, and their weight remains stable.
On the other hand, if a person consumes 2500 calories (energy in) but only expends 2000 calories (energy out), there is a positive energy balance. The excess energy is stored in the body as fat, leading to weight gain over time.
Conversely, if a person consumes 1500 calories (energy in) but expends 2000 calories (energy out), there is a negative energy balance. The body needs to compensate for the energy deficit by utilizing stored energy reserves, such as fat, resulting in weight loss.
Support for the concept of energy balance comes from scientific studies on weight management and obesity. It has been shown that maintaining an energy balance is crucial for weight maintenance, while sustained positive or negative energy balances can lead to weight changes. Additionally, energy balance plays a role in various physiological processes, including metabolism, hormone regulation, and overall health.
By understanding and managing energy balance, individuals can make informed decisions regarding their diet, physical activity, and lifestyle to achieve and maintain a healthy weight and overall well-being.
To know more about energy balance, click here, https://brainly.com/question/31922451
#SPJ11
A buffer is prepared which contains 0.10 m nitrous acid, hno2, and 0.12 m sodium nitrite, nano2. (ka=4.5x10-4) calculate the ph after 0.019 mol of naoh is added to 1.00 l of the buffer.
Answers
0.1 M nitrous acid (HNO2) and 0.12 M sodium nitrite (NaNO2) are combined to create a buffer. (Ka=4.5x10-4) After 0.016 mol of NaOH has been added to 1.00 L of the buffer, determine the pH.
A buffer solution, sometimes referred to as a pH buffer or hydrogen ion buffer, is an aqueous combination of a weak acid and its conjugate base, or vice versa. The pH nitrous changes at all when a small amount of a strong acid or basic is added to it. Buffer solutions are used in a wide range of chemical processes to keep pH values almost constant. Buffering is used by many biological systems to regulate pH in the natural world. For instance, the bicarbonate buffering system regulates the pH of blood.
To learn more about nitrous please click on below link
https://brainly.com/question/17055219
#SPJ4
Why do scientists use microscopes to observe cells?
A. A microscope holds a moving cell very still .
B. A microscope prevents a cell from dividing too quickly.
C. A microscope makes tiny cells appear much larger.
D. A microscope makes a large cell appear smaller.
Answers
Hopefully this was helpful!
If a mixture of 15g of methane, CH4 and 52.5g of oxygen, O2 are ignited, carbon dioxide CO2, one of the products was collected and found the weight 24.75g. What is the percentage yield of the reaction? The chemical equation is: CH4+ 2O2----> CO2+ 2H2O (atomic mass: H=1 C=12 O=16)
Answers
The percentage yield of the reaction is 60%. To find the percentage yield of the reaction, we need to first calculate the theoretical yield of carbon dioxide (CO2).
How do you calculate percentage yield?CH4 + 2O2 → CO2 + 2H2O From the equation, we see that 1 mole of CH4 reacts with 2 moles of O2 to produce 1 mole of CO2. Therefore, we need to calculate the moles of both CH4 and O2 in the given mixture and determine which reactant is limiting the reaction.
The molar mass of CH4 is 12 + 4(1) = 16 g/mol. Therefore, the number of moles of CH4 present in the mixture is:
15 g / 16 g/mol = 0.9375 mol
The molar mass of O2 is 2(16) = 32 g/mol. Therefore, the number of moles of O2 present in the mixture is:
52.5 g / 32 g/mol = 1.6406 mol
According to the balanced equation, 1 mole of CH4 reacts with 2 moles of O2 to produce 1 mole of CO2. Therefore, the maximum number of moles of CO2 that can be produced from the given amounts of CH4 and O2 is:
0.9375 mol CH4 × (1 mol CO2 / 1 mol CH4) = 0.9375 mol CO2
Since 1.6406 mol O2 is more than enough to react with the 0.9375 mol CH4, O2 is in excess and CH4 is the limiting reactant. Therefore, the theoretical yield of CO2 is:
0.9375 mol CO2 × (44 g/mol CO2) = 41.25 g CO2
Now we can calculate the percentage yield of the reaction:
Percentage yield = (actual yield / theoretical yield) × 100%
The actual yield of CO2 is given as 24.75 g. Therefore, the percentage yield is:
(24.75 g / 41.25 g) × 100% = 60%
To know more about percentage yield visit:-
brainly.com/question/30579872
#SPJ1
magenesuim 48.6g + oxygen 32.0 g = magenesuim oxide 80.6 g what is the mass of reactant
Answers
Answer:
2Mg+O=2MgO
Explanation:
32gram of reacting
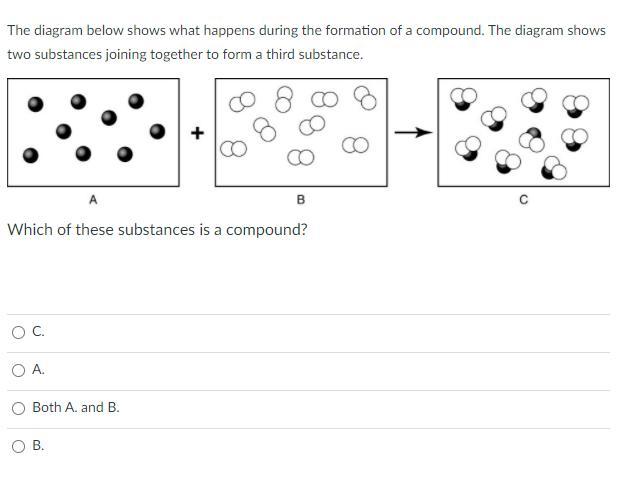
Observe the image of hydrogen chloride. List the properties you see.

Answers
Explanation:
The properties of the hydrogen chloride:
It is a liquid at the given temperature. It is clear, colorless and transparent. It has definite volume which can be taken from the reading on the flask. The shape cannot be easily ascertained. Physical substances as this are called liquids.Please help
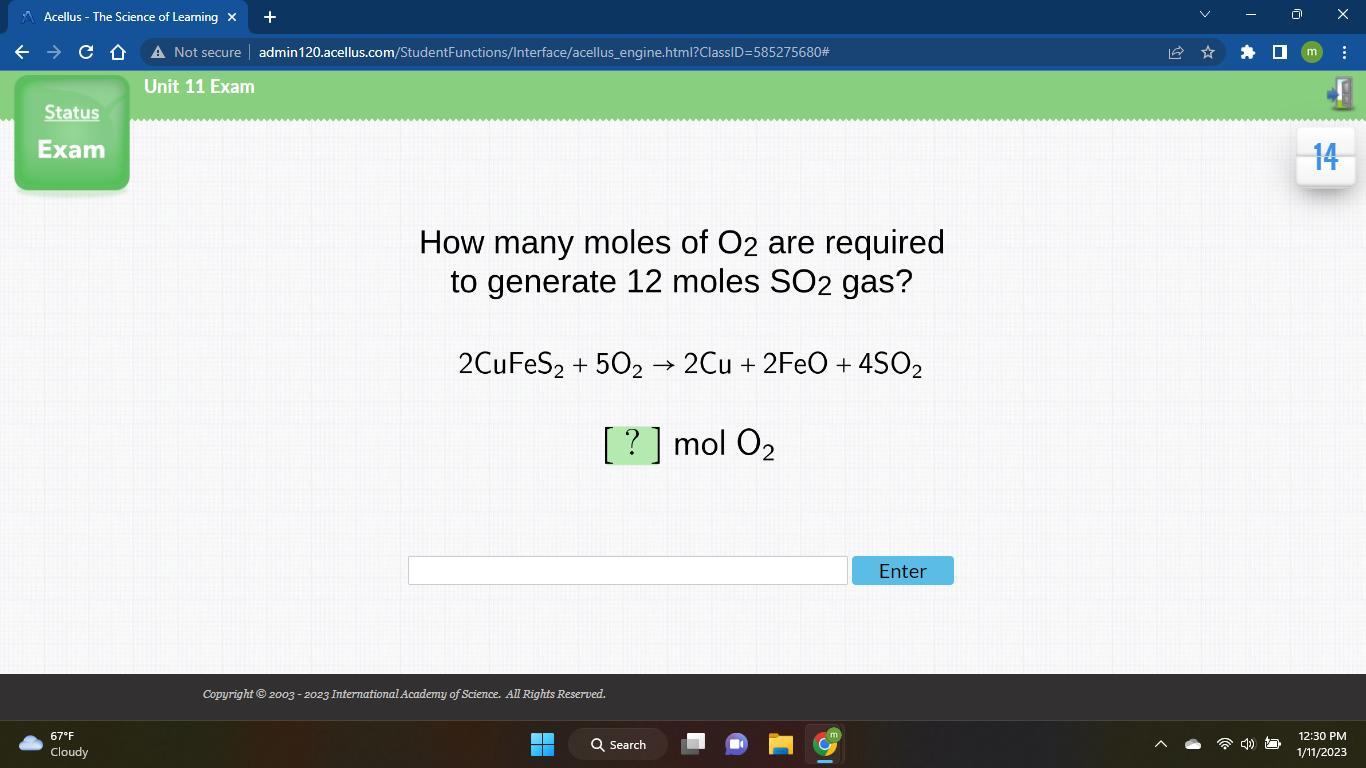
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
Which of the following diagrams has correctly shaded the metals in blue?

Answers
1) Groups of elements in the periodic table.
Metals are located in the middle and left side of the periodic table.
The second picture has correctly shaded the metals.
.

Which solution has the greatest number of hydroxide ions?
i need a friend....
Answers
Answer:
homogeneous solutions
What happens during the rock cycle?
Answers
50 points Acids & Bases
please help me children

Answers
Answer:
See below ↓↓↓↓
Explanation:
1)
a)
HCl + NaOH → NaCl + H₂Ob)
HNO₃ + KOH → KNO₃ + H₂Oc)
Mg(OH)₂ + H₂CO₃ → MgCO₃ + 2H₂Od)
Al(OH)₃ + 3HCl → AlCl₃ + 3H₂Ocalculate the volume of a gas in l at a pressure of 1.00 x10^2 kpa if its volume at 1.2 x 10^2 is 1.50 x 10^3
Answers
The volume of the gas at a pressure of 1.00 x 10^2 kPa is 1.8 x 10^3 L.
To calculate the volume of a gas at a different pressure, we can use Boyle's Law, which states that the product of pressure and volume is constant for a given amount of gas at a constant temperature. Mathematically, it is represented as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Given:
Initial pressure (P1) = 1.2 x 10^2 kPa
Initial volume (V1) = 1.50 x 10^3 L
Final pressure (P2) = 1.00 x 10^2 kPa
We need to find the final volume (V2). Using Boyle's Law formula:
P1V1 = P2V2
(1.2 x 10^2 kPa)(1.50 x 10^3 L) = (1.00 x 10^2 kPa)(V2)
Solving for V2:
V2 = [(1.2 x 10^2 kPa)(1.50 x 10^3 L)] / (1.00 x 10^2 kPa)
V2 = (1.8 x 10^5) / (1.0 x 10^2)
V2 = 1.8 x 10^3 L
So, the volume of the gas at a pressure of 1.00 x 10^2 kPa is 1.8 x 10^3 L.
Visit here to learn more about Boyle's Law, : https://brainly.com/question/30367133
#SPJ11
8
с
Which of the following statements is FALSE about the diagrams above?
Diagram B is a mixture of compounds.
Diagram C is a pure substance of a diatomic element.
Diagram D is a mixture of elements and compounds.
Diagrams A&C represent a pure substance of elements.
14 15

Answers
Answer:
Diagram B is a mixture of compounds
Explanation:
The false statement about any of the diagram is that diagram B is a mixture of compounds.
Diagram is not a mixture of compounds. It is a single compound in gaseous state .
A compound is a combination of elements in a definite grouping. The properties of the compound is distinct from that of the element that combines to form it. Therefore, since we just a black and yellow circle, the diagram is a compound and nothing more. The other descriptions are perfect.Which of the following statements concerning mixtures is correct?
a. The composition of a homogeneous mixture cannot vary.
b. A homogeneous mixture can have components present in two physical states.
c. A heterogeneous mixture containing only one phase is an impossibility
d. More than one correct response..
Answers
The correct option from the given statements concerning mixtures is (d) more than one correct response.
The statement (a) "The composition of a homogeneous mixture cannot vary" is incorrect as the composition of a homogeneous mixture can vary. For example, a mixture of salt and water is homogeneous and its composition can vary depending on the amount of salt and water mixed in it.
The statement (b) "A homogeneous mixture can have components present in two physical states" is correct. Homogeneous mixtures are mixtures that are uniform throughout their composition, meaning that there is no visible difference between the components of the mixture. For example, a mixture of ethanol and water is homogeneous and its components are present in two physical states (liquid and liquid).
The statement (c) "A heterogeneous mixture containing only one phase is an impossibility" is incorrect. A heterogeneous mixture is a mixture where the components are not evenly distributed and the mixture has different visible regions or phases. However, it is possible for a heterogeneous mixture to contain only one phase. For example, a mixture of oil and water is heterogeneous but can have only one phase.
To know more about homogeneous visit:
https://brainly.com/question/30587533
#SPJ11
PLEASE HELP ASAP!!!
What is the mass of the element above?
-1
-2
-3
-4
-5

Answers
Answer:
its 1 not negative
the atom shown below was hydrogen, known as H.
ANSWER THE QUESTION BELOW FOR BRAINLEST AND 10 POINTS
Answers
Answer:
C
Explanation:
It just is, if you look online and conduct research you will see that compounds look just like number 3. It cannot be A or B.
HURRYYY !!!! What radioactive isotope produces Aluminum-13 by beta decay
Answers
Answer:
I DONT KNOW SORRY
Explanation:
Answer:
Iodine-131 is more likely to undergo beta decay than positron decay.
How are amplitude modulation and frequency modulation techniques similar?
Both are used by cell phones to produce images.
Both transform electrical signals into sound waves.
Both modify the pulse of a carrier wave.
Both are used by radio stations to transmit sound.
Answers
Answer:
D. Both are used by radio stations to transmit sound
Explanation:
The main difference between both modulations is that in frequency modulation, the frequency of the carrier wave is modified as per the transmit data, while in amplitude modulation, the carrier wave is modified according to the data. because of this it can NOT be A, B, or C but it CAN be D
PLZ MARK AS BRAINLIEST :)
The amplitude modulation and frequency modulation techniques similar as , Both are used by radio stations to transmit sound.
So, option D is correct one.
What is difference between amplitude modulation and frequency modulation techniques ?The primary distinction between the two types of modulation is that , amplitude modulation modifies the carrier wave in accordance to data, frequency modulation modifies the carrier wave's frequency according to the transmit.The frequency modulation operates between 88 and 108 MHz and the amplitude modulation operates between 535 and 1705 KHz.learn about amplitude modulation
https://brainly.com/question/13265507
#SPJ2
How much energy does it take to boil 100 mL of water? (Refer to table of constants for water. )
A. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × 6. 03 kJ/mol = 33. 5 kJ
B. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × (–285. 83 kJ)/mol = –1586 kJ
C. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × 40. 65 kJ/mol = 226 kJ
D. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × 4. 186 kJ/mol = 23. 2 kJ
Answers
Therefore, it takes approximately 23.2 kJ of energy to boil 100 mL of water.
The correct answer is D. 100 mL × 1g divided by 1mL × 1mol divided by 18.02g × 4.186 kJ/mol = 23.2 kJ
To calculate the energy required to boil 100 mL of water, we need to use the specific heat capacity of water, which is approximately 4.186 J/g·°C. The molar mass of water is 18.02 g/mol.
First, we convert the volume of water from milliliters to grams:
100 mL × 1 g/1 mL = 100 g
Then, we calculate the number of moles of water:
100 g × 1 mol/18.02 g = 5.548 mol
Finally, we multiply the number of moles by the molar heat of vaporization of water, which is approximately 40.65 kJ/mol:
5.548 mol × 4.186 kJ/mol = 23.2 kJ
Therefore, it takes approximately 23.2 kJ of energy to boil 100 mL of water.
Learn more about energy
https://brainly.com/question/8630757
#SPJ11
What is the total number of electrons being shared in the single bonds between Hydrogen and Oxygen atoms in the compound of H2O?
Answers
The total number of electrons being shared in the single bonds between hydrogen and oxygen atoms in H₂O is 2 pairs or 4 electrons.
In the compound H₂O, which represents a molecule of water, there are two single bonds between the hydrogen (H) and oxygen (O) atoms. Each single bond consists of a pair of electrons being shared between the bonded atoms.
Since there are two single bonds in H₂O, there are a total of two pairs of electrons being shared. Therefore, the total number of electrons being shared in the single bonds between hydrogen and oxygen atoms in H₂O is 2 pairs or 4 electrons.
Learn more about Water from the link given below.
https://brainly.com/question/28465561
#SPJ4
I need help, please. ASAP anyone willing to help a port innocent child like me

Answers
Answer:
Kₑq = [H₂]² [O₂] / [H₂O]²
Explanation:
We'll begin by defining equilibrium constant for a reaction. This is given below:
The equilibrium constant for a given reaction is defined as the ratio of the concentration of the products raised to their coefficient to the concentration of the reactants raised to their coefficient.
With the above information in mind, we shall determine the equilibrium constant for the reaction given in the question above. This is illustrated below:
2H₂O (g) <=> 2H₂ (g) + O₂ (g)
Kₑq = [H₂]² [O₂] / [H₂O]²