You are riding a bicycle. If you apply a forward force of 125 N, and you and
the bicycle have a combined mass of 82 kg, what will be the forward
acceleration of the bicycle? (Assume there is no friction. )
O A. 1. 52 m/s2
B. 1. 67 m/s2
C. 3. 37 m/s2
D. 0. 66 m/s2
Answers
The forward acceleration of the bicycle if you apply a forward force of
125 N is 1.52 m/s² option A.
A force is an action that modifies the velocity of an object with mass. It can be a push or a pull and always has magnitude and direction, making it a vector quantity. The unit of force used to quantify it is newtons (N), which is represented by the symbol F.
In its original form, Newton's second law states that the net force acting on an object is equal to the rate of change of its momentum over time. According to this rule, the acceleration of an object is inversely proportional to its mass if the mass of the object is constant, directly proportional to the net force acting on the object, and in the direction of the net force.
Force = 125N
Mass combined = 82kg
Acceleration of the bicycle = x
From Newton second law of motion suggests that:
Force = mass x acceleration
Acceleration = force/mass
= 125/82
= 1.52 m/s²
Therefore, acceleration of the bicycle is 1.52 m/s².
Learn more about Acceleration:
https://brainly.com/question/23722042
#SPJ4
Related Questions
How many grams of KI are dissolved in 250 grams of 20% solution?
Answers
Answer:
180 g
Explanation:
The density of aluminum is 2.7 g/ml. What is the volume of 8.1 g?
Answers
Answer:
volume= 3
Explanation:
D=m/v
2.7=8.1/v
2.7v=8.1
8.1/2.7= 3
8.1/3= 2.7 which is the density!
Answer:
3 mL
Explanation:
\(V = \frac{8.1 g}{3 mL} = 2.7 g/mL\)
What is the balanced equation of Iodine and Starch?
Answers
The balanced equation of Iodine and Starch complex during the test for starch is I₂ + I⁻ → I₃⁻.
Iodine starch complexWhen iodine is added to a starch, a deep blue solution is formed due to the presence of Amylose in starch.
Iodine -potassium reagent is often for the test of starch because iodine is not very soluble in water, thus the dissolution of iodine in water is enabled by the presence of potassium.
Balanced equation of Iodine and StarchI₂ + I⁻ → I₃⁻
Thus, the balanced equation of Iodine and Starch complex during the test for starch is I₂ + I⁻ → I₃⁻.
Learn more about test for starch here: https://brainly.com/question/1698910
#SPJ1
What was the earliest life form on Earth?
A. Protists
B. Bacteria
C. Viruses
Answers
Answer:
a protists
Explanation:
Explain how you could distinguish, without a flame test, between a solution that contains both Mg2+ and Ca2+ from one that contains only Mg2+.
Answers
One way to distinguish between a solution that contains both Mg²⁺ and Ca²⁺ from one that contains only Mg²⁺ without a flame test is by using a precipitation reaction.
A common reagent used for this purpose is sodium phosphate (Na₃PO₄), which reacts with Ca²⁺ions to form a white precipitate of calcium phosphate (Ca₃(PO₄)₂). Therefore, if a solution contains both Mg²⁺ and Ca²⁺, adding sodium phosphate to the solution will result in the formation of a white precipitate. On the other hand, if a solution contains only Mg²⁺, adding sodium phosphate will not result in any visible precipitate. By observing the presence or absence of a precipitate, we can distinguish between the two solutions.
To learn more about flame test https://brainly.com/question/22909071
#SPJ11
Can someone help me here please i need this :(
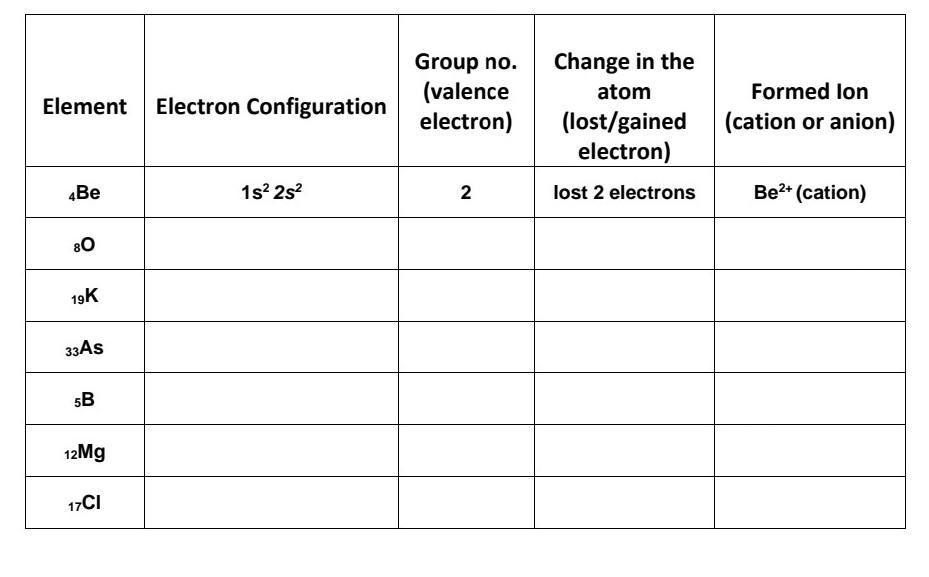
Answers
what more accurate model of the atom emerged from Albert Einstein's contributions and quantum mechanics
Answers
Answer:
Einstein also in 1905 mathematically proved the existence of atoms, and thus helped revolutionize all the sciences through the use of statistics and probability. Atomic theory says that any liquid is made up of molecules (invisible in 1905)
Explanation:
Answer:
The discoveries of Albert Einstein led to development in the understanding of the atom through the study of quantum mechanics and the electron cloud. In this model, there is still a nucleus with protons and neutrons. But unlike the Bohr model, the electrons exist in a cloud outside the nucleus, much like the fruit around the pit of a peach.
Explanation:
Which statement best describes a relationship amongst hypotheses, theories, and laws
Answers
The relationship between hypotheses, laws and theories is that a law is used to explain hypothesis and theories.
What is a law, a theory and hypothesis?A hypothesis is a tentative conjecture explaining an observation, phenomenon or scientific problem that can be tested by further investigation or experimentation.
A theory is a coherent statement or set of ideas that explains observed facts or phenomena and correctly predicts new facts or phenomena not previously observed.
A law is a statement whose sequence or relationship of phenomena is invariable under certain conditions.
This suggests that a law can be used to explain a theory and hypothesis.
Learn more about laws and theories at: https://brainly.com/question/9266219
#SPJ1
Name and draw skeletal formula of all the structural isomers of C4 H10 O that are alcohols.
Answers
One of the isomers of C4H10O that is an alcohol is the 1-butanol or butyl alcohol:
Another of the alcohols that has the given formula is tert-butanol or tert-butyl alcohol:
The third isomer of C4H10O is 2-butanol:
And the last isomer is isobutanol or isobutyl alcohol:




How many moles of calcium,Ca are in 5. 00 g of calcium ??
Answers
There are 0.1247 moles of calcium in 5.00 g of calcium.
The formula to calculate the number of moles is:
moles = mass (in grams) / molar mass
Substituting the values we have:
moles of calcium = 5.00 g / 40.08 g/mol
moles of calcium = 0.1247 mol
A mole is a unit of measurement that represents a certain number of particles. Specifically, one mole of a substance contains Avogadro's number of particles, which is approximately 6.02 x 10^23. These particles can be atoms, molecules, ions, or any other type of particle that can exist in a chemical system.
The concept of moles is important because it allows chemists to easily convert between the mass of a substance and the number of particles it contains. This is because the molar mass of a substance, which is the mass of one mole of that substance, is equal to the sum of the atomic masses of all the atoms in one molecule of that substance. This means that if you have 18 grams of water, you have one mole of water, and if you have any other mass of water, you can easily calculate how many moles of water you have using the molar mass.
To learn more about Moles visit here:
brainly.com/question/31597231
#SPJ4
Select the atomic models that belong to the same element.
YOU MUST PICK TWO!

Answers
Answer:
Model 2 and model 3
Explanation:
Both atomic models represent hydrogen, which consists of one proton in the nucleus. Both atomic models consists of 1 proton, which means that both their atomic numbers are equal to 1
You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false. (a) The freezing point of the solution is unchanged by addition of the solvent.
Answers
False. The addition of a solute to a solvent typically lowers the freezing point of the solution compared to the freezing point of the pure solvent.
This is due to the fact that the solute particles interfere with the arrangement of the solvent particles, which makes it more difficult for the solution to freeze. As a result, the solution has to be cooled to a lower temperature in order for it to freeze, which results in a lower freezing point compared to the pure solvent. Freezing point depression is a colligative property, which means that it depends on the concentration of solute particles in the solution, rather than on their chemical nature. When a non volatile solute (a solute that does not evaporate at room temperature) is dissolved in a solvent, it increases the number of solute particles in the solution. These solute particles interfere with the arrangement of the solvent particles, making it more difficult for the solvent to freeze. As a result, the solution has to be cooled to a lower temperature in order for it to freeze, which results in a lower freezing point compared to the pure solvent. To summarize, the addition of a non volatile solute to a solvent typically lowers the freezing point of the solution, and therefore the statement "The freezing point of the solution is unchanged by the addition of the solvent" is false.
To learn more about solute refer to this link
https://brainly.com/question/7932885
#SPJ4
How many moles of sodium chloride must enter solution in order to completely precipitate the silver ions in one mole of silver nitrate?
Answers
There are one moles of sodium chloride must enter solution in order to completely precipitate the silver ions in one mole of silver nitrate .
Calculation ,
To find number of moles of sodium chloride , we have to write first balanced chemical equation when sodium chloride reacts with silver nitrate .
So , the balanced chemical equation when sodium chloride reacts with silver nitrate is given as ,
\(NaCl + AgNO3\) → \(NaNO3+ AgCl\)
From the above equation we can conclude that molar ratio is equal to 1:1
It means that one mole sodium chloride is required to react with one mole of silver nitrate .
To learn about sodium chloride please click here ,
https://brainly.com/question/9811771
#SPJ4
At a pH of 7, what groups on a molecule will be deprotonated? Still protonated?
Answers
At pH 7, groups with pKa values above 7 will be deprotonated, those with pKa values below 7 will remain protonated, and those around 7 will be partially ionize.
Why does pH 7 cause histidine to deprotonate?This is due to the side chain of histidine having a pKa value of 6.0. The two acidic amino acids are aspartate and glutamate, and at a physiological pH of 7, they both contain a complete negative charge on their side chains.
Which amino acids are susceptible to deprotonation?Tyrosine and serine are two more amino acids that can be deprotonated at high pH levels, although they mostly reside in their protonated, neutrally charged states at physiological pH levels. Ionizable functional groups can be found in amino acids.
To know more about ionize visit:-
https://brainly.com/question/15182996
#SPJ1
A chemist is identifying the elements present in a sample of seawater. What characteristic of
an element's atoms always determines the element's identity?
Answers
Answer:
the protons
Explanation:
the number of protons is constant for each sample of the element. If the number of protons differs, then it is a different element.
The characteristic of an element's atoms that always determines the element's identity is the number of protons. The correct option is C.
What is an element?A chemical element is a substance that cannot be broken down further through any chemical reaction. Each element's atom contains a distinct number of protons.
A hydrogen atom, for example, has one proton, whereas a carbon atom has six. Ions are created by varying the number of electrons in an element's atom.
The number of protons determines an element's identity. The number of protons cannot be changed without changing the element's identity.
The atomic number increases by one when a proton is added, and the element identity changes.
Color, density, melting point, boiling point, and thermal and electrical conductivity are all properties of elements.
Thus, the correct option is C.
For more details regarding element, visit:
https://brainly.com/question/13025901
#SPJ2
Your question seems incomplete, the missing options are:
The location of valence electrons.
The number of energy levels.
The number of protons.
The number of neutrons.
What geographic obstacle causes water to condense in an air mass as the air mass moves? Explain your answer.
A. Forests
B.oceans
C.prairies
D.mountains
Answers
Answer:
Mountains
Explanation:
Mountains are colder than the lower elevations. When humid air enters a cooler region, the saturated air begins to cool and this reduces the ability of the air to maintain the evaporated water is the gas state. Molecules of water have reduced enerfy at the cooler temperatures. They will slow down and coalesce with other water molecules to form small droplets of water. The atmoshere cannot hold these heaevier droplets and they begin to fall (i.e., rain).
One migh ask why does the air temperature drop at higher altitudes? Heat rises, so why shouldn't it be warmer on top a mountain, compared to the valley. The reason is that the atmosphere becomes less dense the further from Earth's center on gets. This less dense air has a lower water saturation point, so it rains.
When an organism dies it is exposed to many different types of biotic and abiotic factors biotic factors include?
Answers
Answer:
the organisms themselves,other organisms,interactions between living organisms, and even their waste.
You place 696 grams of butane c4 h10 and 2080 grams of oxygen into a reaction chamber which of the reactants is the limiting reactant? How many grams of carbon dioxide and water will be produced? How much of the excess reactant will remain after the limiting reactant is totally consumed?
Show all your work
Answers
The limiting reactant is oxygen. 1760.40 g and 901.00 g of carbon dioxide and water are produced, respectively. 114.8 g of the excess reactant remain after the limiting reactant is totally consumed.
The limiting reactant is the reactant that is completely consumed in a chemical reaction and determines the amount of product formed. To determine the limiting reactant, we need to compare the amount of each reactant present to the amount required by the balanced chemical equation.
The balanced chemical equation for the combustion of butane is:
2 C₄H₁₀ + 13 O₂ → 8 CO₂ + 10 H₂O
From the balanced equation, we can see that 2 moles of butane react with 13 moles of oxygen to produce 8 moles of carbon dioxide and 10 moles of water. We can use the molar masses of each reactant to determine the number of moles present:
moles of butane = 696 g / 58.12 g/mol = 11.98 mol
moles of oxygen = 2080 g / 32.00 g/mol = 65.00 mol
Next, we can compare the amount of each reactant present to the amount required by the balanced equation:
11.98 mol butane / 2 mol = 5.99
65.00 mol oxygen / 13 mol = 5.00
Since the ratio for oxygen is smaller, oxygen is the limiting reactant. We can use the ratio of the limiting reactant to determine the amount of product formed:
5.00 (8 mol CO₂) = 40.00 mol CO₂
5.00 (10 mol H₂O) = 50.00 mol H₂O
We can then use the molar masses of each product to determine the mass of each product formed:
mass of CO₂ = 40.00 mol (44.01 g/mol) = 1760.40 g
mass of H₂O = 50.00 mol (18.02 g/mol) = 901.00 g
Finally, we can use the ratio of the limiting reactant to determine the amount of excess reactant remaining
5.00 (2 mol butane) = 10.00 mol butane
11.97 mol butane - 10.00 mol butane = 1.97 mol butane
1.97 mol butane (58.12 g/mol) = 114.8 g butane
So, the limiting reactant is oxygen, 1760.40 grams of carbon dioxide and 901.00 grams of water will be produced, and 114.8 grams of butane will remain after the limiting reactant is totally consumed.
Learn more about limiting reactant here: https://brainly.com/question/14222359.
#SPJ11
Determine the mass in grams of 2.25mol of Calcium. The molar mass of Calcium is 40.08g/mol
Answers
Answer:
90.18g
Explanation:
1mole—>40.08g of Ca
2.25moles—> ?
2.25×40.08=90.18g
List down the observations made by Rutherford during the gold foil experiment.
Answers
Answer:
1) The volume occupied by an atom is composed of mainly empty space
2) Atoms have a very small, relatively dense, central nucleus that is positively charged
3) The region around the nucleus of an atom are orbited by negatively charged electrons in a the same fashion planets orbit around the Sun.
Explanation:
The selection of gold for the gold foil experiment was due to its ability to be rolled into extremely thin sheets such that it was expected for alpha particle to perforate or pass through the foil.
A block of material has a volume of 2 mº. It has a mass of 1 x 104 kg. Calculate the density of the materia
sample and express your results in g/cm with appropriate scientific notation.
Answers
Answer:
d = 0.5×10¹g/cm³
Explanation:
Given data:
Volume of material = 2 m³
Mass of material = 1×10⁴ Kg
Density of material = ?
Density:
Density is equal to the mass of substance divided by its volume.
Formula
d = m/v
Solution:
Volume of material = 2 m³
1 m³ = 1000000 cm³
2 ×10⁶cm³
Mass of material = 1×10⁴ Kg
1 Kg = 1000 g
1×10⁷ g
by putting values into density formula
d = 1×10⁷ g/2 ×10⁶cm³
d = 0.5×10⁷⁻⁶ g/cm³
d = 0.5×10¹g/cm³
Study the image
A house on a beach (left side) near the ocean (right side). Arrows show movement counterclockwise.
What do the arrows indicate?
Air moves in a direction that creates land breezes.
Air above land warms faster than air above water.
Air blows over long distances in a continual straight path.
Air pressure remains the same while cycling over land and water.
Answers
Answer:
A
Explanation:
Just Because
The arrow shows movement counterclockwise. The arrows indicate air moves in a direction that creates land breezes. The correct option is A.
What is oceanography?The study of the oceans in a whole is known as oceanography. Numerous subjects are covered by oceanography, including marine life and ecosystems, currents and waves, the movement of sediments, and the geology of the ocean floor.
The arrows depict the air's motion as land breezes develop. Land cools far more quickly than water does in coastal regions, which causes the air above it to also cool.
When this occurs, it travels downward and toward the land while becoming denser. Due to the high pressure, winds are forced to blow in the direction of the water (ocean).
Therefore, the correct option is A. Air moves in a direction that creates land breezes.
To learn more about oceanography, refer to the link:
https://brainly.com/question/11274173
#SPJ2
balence the equation : _____ Ga2O3 + _____ Li -----------> ______ Li2O + ______ Ga
Answers
Answer:
Ga2O3 + 6Li ------> 3Li2O + 2Ga
Answer:
1 Ga2O3 + 6Li——-> 3 Li2O + 2 Ga
Explanation:
What is the mass of a piece of silver that when dropped into a 100.0ml graduate cylinder cause the water level to rise from 12.65ml to 18.76 ml?
Answers
Answer:
dgdgdf
Explanation:
and yeah thatds it
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
the mass percentage of hydrochloric acid within a solution is 40.00% . given that the density of this solution is 1.200 g/ml, find the molarity of the solution.
Answers
The molarity of the solution will be 13.12 M.
The formula used to calculate molarity is as follows:
Molarity = Number of moles of solute / Volume of solution
Consider 1L sample of the solution.
So, use the density of the solution to determine grams in 1L,
1.200 g/ML × 1000 mL = 1200g
The percent concentration by mass is 40.0% HCl.
The mass of hydrochloric acid in 1200g of solution,
1200 × 40/100 = 480 g HCl
Use hydrochloric acid's molar mass to determine number of moles,
1 mole HCl = 36.46g
480 g = 13.17 moles HCl
The molarity of the solution will thus be 13.17/1.00 L = 13.17.
To know more about the hydrochloric acid, here
brainly.com/question/15231576
#SPJ4
A rate constant obeys the Arrhenius equation, the factor A 2.2 x 1013 s and the activation energy being 150. kJ mol. What is the value of the rate constant at 227°C, in 6.7x10-22 s-1 b. 2.1x1013 -1 1.5x101 s 4.7x10-3 s1 a. C.
Answers
The rate constant at 227°C is a. 6.7 x \(10^{-22}\).
How to find the rate constant of a reaction?The Arrhenius equation states that the rate constant (k) is equal to A × e(-Ea/RT).
Given values: A = 2.2 x 10¹³ s⁻¹, Activation energy (Ea) = 150 kJ/mol, Temperature (T) = 227°C = 500 K.
For this, we need to substitute the given values in the Arrhenius equation as
k = A × e(-Ea/RT)
k = 2.2 x 10¹³ s⁻¹ × e(-150000 J/mol / (8.31 J/mol-K × 500 K))
k = 2.2 x 10¹³ s⁻¹ × e(-30.12)
k = 6.69 x 10⁻¹² s⁻¹
Therefore, the value of the rate constant at 227°C is 6.69 x 10⁻¹² s⁻¹. Hence, option A is the correct answer.
To know more about Arrhenius equation:
https://brainly.com/question/30653541
#SPJ11
Please help
If you know Spanish I’ll mark mark you as brainlister

Answers
section 5:
1. D
2.E
3.F
4.A
5. C
6. B
Answer:
5 Match each picture with a correct word or expression
1. el premio d.
2. la vuelta a Francia e.
3. los juegos Olímpicos f.
4. soy musculoso a.
5. hacemos ejercicio c.
6. metimos un gol b.
6 Translate the following sentences into spanish:
1. I like watching the olympic games
Me gusta ver los juegos Olímpicos
2. the pan American games are a very important competition
Los juegos Panamericanos son una competición muy importante
3. to stay fit it is good to follow a well balanced diet
Para mantenerse en forma es bueno seguir una dieta sana y equilibrada
4. to score a goal it is necessary to play on a team
Para marcar un gol es necesario jugar en equipo
5. The tour de France is the most famous cycling competition in the world
El Tour de Francia es la competición de ciclismo más famosa del mundo.
6. the world cup is the most popular sporting event in the world
La copa del mundo es el evento deportivo más popular del mundo
I highly recommend to read this carefully.
1. Consider the generic reaction:A + 2BC AH = -55 kJDetermine the amount of heat emitted when each amountof reactant completely reacts (assume that there is morethan enough of the other reactant).(a) 1 mol A(b) 2 mol A(c) 1 mol B(d) 2 mol B
Answers
Answer:
(a) 55 kJ of heat are released when 1 mol of reactant A is used;
(b) 110 kJ of heat are released when 2 moles of reactant A are used;
(c) 27.5 kJ of heat are released when 1 mol of reactant B is used;
(d) 55 kJ of heat are released when 2 moles of reactant B are used.
Explanation:
The question requires us to determine the amount of heat released when the given amounts of reactants are used, considering the following balanced chemical equation:
\(A+2B\rightarrow C\text{ }\Delta H=-55kJ\)When the enthalpy change for a reaction (or heat of reaction, ΔH) is given in units of energy, such as kilojoules (kJ), and not units of energy per mol (such as kJ/mol), we can consider that ΔH corresponds to the heat absorbed or released for the molar quantities of reactants as given in the balanced chemical equation. In the case given by the question, for example, we can say that 55 kJ of energy are released when 1 mol of A reacts with 2 moles of B.
Therefore, we can use the molar quantitites from the balanced chemical equation as a reference to determine the amount of heat released when different amounts of reactants are used.
Considering the information above, we can calculate:
(a) heat released when 1 mol of A reacts:
Note that 1 mol of A corresponds to the amount of reactant A given by the balanced chemical equation. Therefore, 55kJ of energy are released when 1 mol of A is used.
(b) heat released when 2 moles of A reacts:
Note that 2 moles of A corresponds to the double of the amount of reactant A given by the balanced chemical equation. Thus, we must multply ΔH by 2: 55 kJ x 2 = 110 kJ of energy are released when 2 moles of A are used.
(c) heat released when 1 mol of B reacts:
Note that 1 mol of B corresponds to half of the amount of reactant B given by the balanced chemical equation. Thus, we must divide ΔH by 2: 55 kJ / 2 = 27.5 kJ of energy are released when 1 mol of B is used.
(d) heat released when 2 moles of B reacts:
Note that 2 moles of B corresponds to the amount of reactant B as given by the balanced chemical equation. Therefore, 55 kJ of energy are released when 2 moles of B are used.
What are the four properties of mixtures?.
Answers
Four properties of mixture are- it's constitution is present in any ratio, energy is neither produced or evolved, no fixed melting or boiling point, components can be seperated.
A mixture is composed of two or more simpler substances. These can be compounds or chemical elements. There are 2 types of mixtures- homogeneous and heterogeneous. Four properties of a mixture-
1. The constituents of mixture can be present in any ratio and has no fixed composition.
2. Energy is neither produced nor evolved to form mixture.
3. The mixture has no fixed boiling points and melting points.
4. Components of mixtures can be separated by physical methods simply.
To learn more about mixture visit the link- https://brainly.com/question/2331419
#SPJ4