Answers
- Kc for the a) reaction:
\(Kc=\frac{\lbrack CO\rbrack^2}{\lbrack CO_2\rbrack^{}}\)- Kc for the b) reaction:
\(Kc=\frac{\lbrack HI\rbrack^2}{\lbrack H_2S\rbrack}\)- Kc for the c) reaction:
\(Kc=\lbrack O_2\rbrack\)To write the expression for the equilibrium constant (kc), it is important to remember that:
- The concentration of the products is multiplied in the numerator, while the concentration of the reactants is multiplied in the denominator.
- Only the concentrations of the gaseous and/or dissolved species should be included.
Related Questions
2.50 g of As2O3 are titrated with 38.5 mL of KMnO4 to reach the end point.
5As2O3(s)+4MnO−4(aq)+9H2O(l)+12H+(aq)⟶10H3AsO4(aq)+4Mn2+(aq)
Calculate the concentration of the KMnO4 solution.
Answers
50 g of As\(_2\)O\(_3\) are titrated with 38.5 mL of KMnO\(_4\) to reach the end point. 0.26M is the concentration of the KMnO\(_4\) solution.
Concentration in chemistry refers to the quantity of a material in a certain area. The ratio of the solute within a solution to the solvent or whole solution is another way to define concentration. In order to express concentration, mass in unit volume is typically used.
The solute concentration can, however, alternatively be stated in moles or volumetric units. Concentration may be expressed as per unit mass rather than volume.
5As\(_2\)O\(_3\)(s)+4MnO\(_4\)⁻(aq)+9H\(_2\)O(l)+12H⁺(aq)⟶10H\(_3\)AsO\(_4\)(aq)+4Mn\(_2\)⁺(aq)
the stoichiometry ratio between As\(_2\)O\(_3\) and MnO\(_4\)⁻ is 5:4
0.0126 moles of As\(_2\)O\(_3\) will react with 4/5×0.0126 moles = 0.01008moles
0.01008moles of MnO\(_4\)⁻ is present in 38mL
concentration of KMnO\(_4\)= moles×volume
= 0.010/38×1000
=0.26M
To know more about Concentration, here:
https://brainly.com/question/10725862
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
HELP WHAT NUMBER IS THIS??
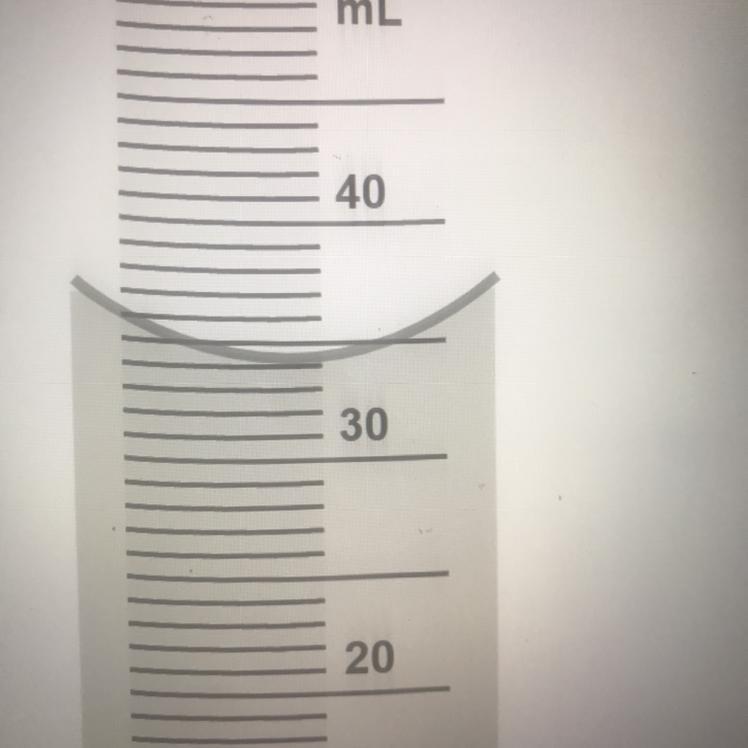
Answers
Can you guys answer question 4 on new substance for Chemistry tysm

Answers
The answer is a bevasue it then becomes a chemical compound
Answer:
a
Explanation:
this would result in a compound and compounds are chemical changes so i think im right....
Describe an autopsy table
Answers
After the body is cleaned, the body is weighed and measured before being placed on the autopsy table for reexamination. The autopsy table is typically a slanted, aluminum table with raised edges that has several faucets and drains used to wash away collecting blood during the internal investigation.
Answer:
A table used to scientifically determine the cause of death of a human or animal
Explanation:
According to the VSEPR theory, a molecule or ion of CO2 will have a _______ shape. A. flat linear B. flat trigonal C. bent D. pyramidal E. None of the Above
Answers
According to the VSEPR theory, a molecule or ion of CO2 will have a flat linear shape. Option A
In CO2, the carbon atom forms double bonds with each oxygen atom. The carbon-oxygen double bonds consist of two pairs of electrons, which are arranged linearly, leading to a linear molecular shape.
The VSEPR theory suggests that electron pairs in the valence shell of the central atom repel each other and try to position themselves as far apart as possible, resulting in the linear shape.
The VSEPR theory allows us to predict the molecular geometry based on the arrangement of bonding and non-bonding electron pairs around the central atom. In the case of CO2, there are no lone pairs of electrons on the carbon atom, and the molecule has a symmetrical arrangement, leading to a linear shape. Option A
For more such questions on VSEPR theory visit:
https://brainly.com/question/14225705
#SPJ8
after 65 minutes, the amount of a particular radioisotope remaining is 3.13% of the initial amount. what is the half life of the radioisotope?
Answers
Answer:
13 mins
Explanation:
We need to find how many half lives are required to reach .0313
.0313 = 1/2^n log both sides and solve for n
log.0313 / log (1/2 ) = n where n= NUMBER of halflives
n = ~ 5 half lives
these 5 half lives took up 65 minutes
so EACH half life is 65/5 = 13 mins
Using the following equation, determine the % yield from the following reaction if 3.050E1 g of
octane (C8H18) react with excess oxygen to produce 8.179E1 g of CO₂ (g).
2C8H18 (1) +2502(g) → 16CO2 (g) + 18H₂O(l)
Answers
The percent yield is 86.1%.
How do we obtain the percent yield?The percent yield is a measure of the efficiency of a chemical reaction and is calculated by comparing the actual yield of a reaction to the theoretical yield that could be obtained based on stoichiometry. The percent yield is expressed as a percentage and is calculated using the following formula:
Percent yield = (Actual yield ÷ Theoretical yield) x 100%
We can see;
Number of moles of octane = 3.05 * 10^1/114 g/mol
= 0.27 moles
Then from the reaction equation;
2 moles of octane produces 16 moles of CO2
0.27 moles of octane would produce 0.27 * 16/2
= 2.16 moles
Theoretical yield of the product = 2.16 moles * 44 g/mol
= 95 g
Thus we have that;
% yield = 8.179 * 10^1 g/95 g * 100/1
= 86.1%
Learn more about percent yield:https://brainly.com/question/17042787
#SPJ1
C6H12O6 + 602 → 6CO2 + 6H₂O
The most efficient ratio is
1 C6H12O6 6 02.
Which set of reactants will be the most
efficient (least wasteful of materials) for
the reaction?
A. 1.0 mol C6H12O6 and 3.0 mol O₂
B. 1.5 mol C6H₁2O6 and 3.0 mol O₂
C. 3.0 mol C6H₁2O6 and 6.0 mol O₂
D. 0.5 mol C6H₁2O6 and 3.0 mol O₂
Answers
Answer:
D
Explanation:
The ratio of C6H12O6 (which will be referred to as "the carb") to oxygen is 1 to 6, so if we find an answer which has the same ratio, it should be chosen. A is 1:3
B is even worse with a ratio of the carb to oxygen of 1:2
C is the same as B, 1:2
D has a ratio of the carb to oxygen of 1:6, which is what we are looking for.
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliestCalculate the density of CO2 at a pressure of 685.0 torr and 41.0°C .
R=0.0821 (L*atm)/(mol *K)
Answers
T=41+273=314 k
M=(12)+(16×2)=44g/mol
d=PM/RT
d=685×44/0.0821×314
d=1169.15 g/L
Write a word equation and a skeleton equation for the chemical reaction.
A. Solid iron reacts with aqueous copper(ii) nitrate to produce solid copper and aqueous iron(ii) nitrate.
Answers
Nitrogen is generated by a chemical reaction and 1.80 liters is collected over water at 35.0°C when the pressure in the laboratory is 775 mm Hg. What is the partial pressure (in mm Hg) of nitrogen? The partial pressure of water at this temperature
42.2 mm Hg.
5.45 mm Hg
817 mm Hg
733 mm Hg
18.4 mm Hg
Answers
The partial pressure of nitrogen is approximately 733 mm Hg. Please note that the answer is rounded to the nearest whole number, which is 733 mm Hg. Option D)
To determine the partial pressure of nitrogen, we need to consider the Dalton's Law of Partial Pressures, which states that the total pressure of a gas mixture is the sum of the partial pressures of each gas present.
Given that the total pressure in the laboratory is 775 mm Hg and the partial pressure of water at 35.0°C is 42.2 mm Hg, we can calculate the partial pressure of nitrogen.
To find the partial pressure of nitrogen, we subtract the partial pressure of water from the total pressure:
Partial pressure of nitrogen = Total pressure - Partial pressure of water
Partial pressure of nitrogen = 775 mm Hg - 42.2 mm Hg
Partial pressure of nitrogen = 732.8 mm Hg
Therefore, the partial pressure of nitrogen is approximately 733 mm Hg.
Please note that the answer is rounded to the nearest whole number, which is 733 mm Hg. Option D) is correct.
For more question on pressure
https://brainly.com/question/24719118
#SPJ8
An aqueous solution known as Ringer's lactate is administered intravenously to trauma victims suffering from blood loss or severe burns. The solution contains the chloride salts of sodium, potassium, and calcium and is also 3.75 mM in sodium lactate (NaC3H5O3).
How many grams of sodium lactate are needed to prepare 8.50 liters of Ringer's lactate?
Answers
8.50 liters or Ringer's lactate require 4.5 g of sodium lactate to make.We learn from the question that the solution has 4.75 mM of sodium lactate in it (NaC3H5O3).
What is the Ringer's lactate aqueous solution?Trauma sufferers with severe burns or blood loss are given an intravenous dose of Ringer's lactate, an aqueous solution.The mixture also has 3.25 mM sodium lactate and the sodium, potassium, & calcium chloride salts (NaC3H5O3).
What is the purpose of Ringer's solution?For burn and trauma victims, this intravenous solution is used to quickly increase the volume of circulating blood.Also, it is applied to patients with a wide range of medical disorders and during surgery.
To know more about solution visit:
https://brainly.com/question/30665317
#SPJ1
Why are the waters of the North Atlantic, like the waters near coral reefs, murky green? 1. The color is caused by chlorophyll in the water.
2. The color is caused by the waste products given off by fish.
3. The color is caused by coral reefs in the area.
4. The color is caused by the lack of oxygen in the water
Answers
The waters of the North Atlantic are murky green because the color is caused by coral reefs in the area. That is option 4.
The effects of overgrowth of algaeThe excess nitrogen and phosphorus which is found in fertilizers can cause an overgrowth of algae in a short period of titme, when washed into water bodies. This is called algae blooms.
The effects of overgrowth of algae on aquatic bodies include the following:
reduce the ability of fish and other aquatic life to find food It causes thick, green muck that impacts clear water, recreation, businesses and property values.Therefore, the murky green color is caused by coral reefs in the area.
Learn more about coral reefs here:
https://brainly.com/question/10770235
Answer:
The waters of the North Atlantic are murky green because the color is caused by coral reefs in the area. That is option 4.
The effects of overgrowth of algae
The excess nitrogen and phosphorus which is found in fertilizers can cause an overgrowth of algae in a short period of titme, when washed into water bodies. This is called algae blooms.
The effects of overgrowth of algae on aquatic bodies include the following:
reduce the ability of fish and other aquatic life to find food
It causes thick, green muck that impacts clear water, recreation, businesses and property values.
Therefore, the murky green color is caused by coral reefs in the area.
Learn more about coral reefs here:
brainly.com/question/10770235
Explanation:
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
is energy from the light source conduction convention or radiation
Answers
g Five calcite, CaCO3 (MW 100.085 g/mol), samples of equal mass have a total mass of 12.3±0.1 g. What is the absolute uncertainty (grams) of calcium in each average calcium mass of the sample? Assume that the relative uncertainties in atomic mass are small compared the uncertainty of the total mass.
Answers
Answer:
The value is \(L = 0.985 \pm 0.00801 \ g\)
Explanation:
From the question we are told that
The molar mass of \(CaCO_3\) is \(MW = 100.085 \ g/mol\)
The total mass is \(m_g = 12.3 \ g\)
The uncertainty of the total mass is \(\Delta g = 0.1\)
Generally the molar weight of calcium is \(M_c = 40 g/mol\)
The percentage of calcium in calcite is mathematically represented as
\(C = \frac{40.07}{100.085} * 100\)
\(C = 40.03 \% \)
Generally the mass of each sample is mathematically represented as
\(m= \frac{m_g}{5}\)
\(m= \frac{12.3}{5}\)
\(m= 2.46 \ g \)
Generally mass of calcium present in a single sample is mathematically represented as
\(m_c = 2.46 * \frac{40.04}{100}\)
\(m_c = 0.985 \ g \)
The uncertainty of mass of a single sample is mathematically represented as
\(k = \frac{\Delta g }{5}\)
\(k = \frac{0.1 }{5}\)
\(k = 0.02\ g \)
The uncertainty of mass of calcium in a single sample is mathematically represent
\(G = \frac{0.02 * 40.04}{ 100}\)
\(G = 0.00801 \ g \)
Generally the average mass of calcium in each sample is
\(L = 0.985 \pm 0.00801\)
If you want there to be less of you tomorrow than today... you need to lose
Answers
Answer:
rise your words, not your voice rain grows flowers thunder does not.
Explanation:
off the top of my head
Define biotechnology. } List two advantages in the use of biotechnology
Answers
Advantages of biotechnology:
Improvement of plants and animal breeds to give a high yield of their products.
Pests and pathogen control in agriculture which will reduce the loss of yield in food crops.
Synthesis of biocatalyst which can be used for enhancing the reactions which can be carried out in vitro or laboratory conditions.
Sewage treatment or water recycling can be done with the help of transgenic microbes which have better efficiency and speed.
Biotechnology is the use of living organisms or other biological systems in the manufacture of drugs or other products or for environmental management, as in waste recycling: includes the use of bioreactors in manufacturing, microorganisms to degrade oil slicks or organic waste, genetically engineered bacteria to produce human hormones, and monoclonal antibodies to identify antigens.
Biotech offers the possibility of improving human health, the environment, and agriculture while creating more sustainable modes of production.
Please help!!! Your reading assignment lists possible errors that can occur when conducting an experiment. Which of the following would be a possible error?
a) Humans making exact measurements when conducting experiments.
b) Humidity and temperature in the room may influence equipment
c) Making sure all equipment is clean and calibrated (carefully adjusted)
Answers
Answer:
The answer is B.
Explanation:
Systematic error arises from a faulty measuring device, imperfect observation methods, or an uncontrolled environment.
Answer:
your answer is a
Explanation:

the table below gives the atomic number of elements w x and y and z.The the letters do not represent the actual symbols of the elements .
W. X Y. Z
9. 10. 11. 12
which one of the element is less reactive explain .
Answers
Element w is less reactive than elements x, y, and z. The element with the lower atomic number is typically less reactive.
Element w has an atomic number of 9, element x has an atomic number of 10, element y has an atomic number of 11, and element z has an atomic number of 12. Based on this information, we can conclude that element w is less reactive than elements x, y, and z.
This is because the reactivity of an element is largely determined by the number of valence electrons it has. Valence electrons are the electrons in the outermost shell of an atom that are involved in chemical reactions. Elements with fewer valence electrons are less reactive because they are more stable. Element w has only one valence electron, while elements x, y, and z have two, three, and four valence electrons, respectively.
In general, elements with a full outermost shell of electrons, such as the noble gases, are the least reactive because they are highly stable. Elements that are close to having a full outermost shell, such as element w, are also relatively stable and less reactive. On the other hand, elements with only a few valence electrons, such as the alkali metals, are highly reactive because they are trying to gain or lose electrons in order to achieve a full outermost shell.
Overall, the reactivity of an element is determined by its electronic structure, with elements having fewer valence electrons generally being less reactive than those with more. In the case of the elements w, x, y, and z, we can see that element w has the fewest valence electrons and is therefore the least reactive.
For more such questions on Element
https://brainly.com/question/28376204
#SPJ11
What doesn’t change the resistance of a wire
Answers
The factor that doesn’t change the resistance of a wire is pressure. option A.
What is resistance of a wire?Resistance is a conductor's capacity to thwart the passage of current. It is controlled by the interplay of the applied voltage and the electric current passing through it. The amount of opposition any object applies to the flow of electric current is referred to as resistance.
The ohm, a unit of measurement for resistance, is represented by the Greek letter omega. According to Ohm's law, the voltage across two places is precisely proportional to the current flowing through a conductor between them.
Hence option A is correct.
Learn more about resistance at
https://brainly.com/question/17563681
#SPJ1
missing part;
The pressure
The length of the resistor.
The thickness of the resistor.
The temperature of the conductor.
Zn+HNO3 --> Zn(NO3)2+H2
PLS ANSWER IT FAST I REALLY NEED IT!!!!
Answers
The given equation represents the reaction between zinc (Zn) and nitric acid (HNO3). The balanced chemical equation for this reaction is : Zn + HNO3 → Zn(NO3)2 + H2Zinc is a metal, and nitric acid is an acid.
This reaction is a redox reaction as the oxidation state of Zinc is changed from 0 to +2, and the oxidation state of Nitrogen in Nitric acid is changed from +5 to +4.The reactants in the equation are zinc and nitric acid. Zinc is a solid metal, while nitric acid is a colorless, corrosive liquid. In this reaction, zinc reacts with nitric acid to form zinc nitrate and hydrogen gas. Zinc nitrate is a white crystalline substance that dissolves in water easily. Hydrogen gas is a colorless, odorless gas.The balanced chemical equation for this reaction is derived by ensuring that the total number of atoms of each element in the reactants is equal to the total number of atoms of the same element in the products. The coefficients in front of each substance show the number of atoms or molecules of each substance needed for the reaction to occur.In this case, one atom of zinc reacts with one molecule of nitric acid to form one molecule of zinc nitrate and one molecule of hydrogen gas.
The reaction between zinc and nitric acid is an exothermic reaction as heat is released during the reaction.The reaction between zinc and nitric acid is an important reaction as it is used in the production of zinc nitrate, which is used in the manufacture of other zinc compounds.
for such more questions on equation
https://brainly.com/question/28818351
#SPJ8
1.547 grams of hydrated MgSO4 is heated in a crucible. After heating, 0.7554 grams of anhydrous MgSO4 remains in the crucible. How many waters of hydration were attached to the MgSO4
Answers
Answer:
7
Explanation:
Let x represent the number of moles of water in the hydrated salt i.e MgSO₄.xH₂O
The following data were obtained from the question:
Mass of MgSO₄.xH₂O = 1.547 g
Mass of anhydrous MgSO₄ = 0.7554 g
Mole of H₂O = x =?
Next, we shall determine the mass of water, H₂O in the hydrated salt, MgSO₄.xH₂O. This can be obtained as follow:
Mass of MgSO₄.xH₂O = 1.547 g
Mass of anhydrous MgSO₄ = 0.7554 g
Mass of H₂O =?
Mass of H₂O = (Mass of MgSO₄.xH₂O) – (Mass of anhydrous MgSO₄)
Mass of H₂O = 1.547 – 0.7554
Mass of H₂O = 0.7916 g
Finally, we shall determine the value of the x as illustrated below:
Mass of MgSO₄.xH₂O = 1.547 g
Molar mass of MgSO₄.xH₂O = 24 + 32 + (16×4) + x[(2×1) + 16]
= 24 + 32 + 64 + x(2 + 16)
= 120 + 18x
Mass of H₂O = 0.7916 g
Molar mass of xH₂O = 18x
Molar Mass of xH₂O/ Molar mass of MgSO₄.xH₂O = mass of xH₂O /Mass of MgSO₄.xH₂O
18x/ 120 + 18x = 0.7916/1.547
Cross multiply
0.7916 (120 + 18x) = 18x × 1.547
94.992 + 14.2488x = 27.846x
Collect like terms
94.992 = 27.846x – 14.2488x
94.992 = 13.5972x
Divide both side by 13.5972
x = 94.992 / 13.5972
x = 7
Thus, the formula for the hydrated salt, MgSO₄.xH₂O is MgSO₄.7H₂O
Number of moles of water, H₂O in the hydrated salt MgSO₄.7H₂O is 7.
The number of moles of attached water molecules is 7.
Mass of hydrated MgSO4 = 1.547 grams
Mass of anhydrous MgSO4 = 0.7554 grams
Number of moles of hydrated MgSO4 = 1.547 grams/120 + 18x
Number of moles of anhydrous MgSO4 = 0.7554 grams /120
Number of moles of anhydrous salt = Number of moles of hydrated salt
0.7554 grams /120 = 1.547 grams/120 + 18x
0.7554(120 + 18x) = 1.547 × 120
90.6 + 13.6x = 185.6
185.6 - 90.6 /13.6 = x
x = 7
The number of moles of attached water molecules is 7.
Learn more about water molecules:https://brainly.com/question/1195122
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8
what are the impact of soil Science to the development of Ghana's agriculture?
Answers
In an ecosystem, the impact of soil Science to the development of Ghana's agriculture is that it provides suitable conditions for root germination and growth.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ9
Balance the equations by inserting coefficients as needed.
equation 1:
CaCO3 + HCl -> CaCl2 + CO2 + H2O
CaCO3+HCl⟶CaCl2+CO2+H2O
equation 2:
C6H12O2 + O2 -> CO2 + H2O
C6H12O2+O2⟶CO2+H2O
Answers
Answer:
1. CaCO3 + 2HCl → CaCl2 + H2O + CO2
2. C6H12O2 + 8O2 → 6CO2 + 6H2O
Explanation:
The balanced chemical equation is (i) CaCO3+2HCl⟶CaCl2+CO2+H2O
(ii) C6H12O2+ 8O2⟶CO2+6H2O
What is balanced chemical equation?An equation with equal amounts of every atom of an element on both endpoints of the arrow was called a balanced equation.
Given chemical equation is:
(i) CaCO3+HCl⟶CaCl2+CO2+H2O
It can be seen that in left side of the chemical equation count of chlorine atom is one while right side of the chemical equation it is two. So, by multiplying 2 as a coefficient in the right side of the equation. Balanced chemical equation will be
CaCO3+2HCl⟶CaCl2+CO2+H2O
(ii) C6H12O2+O2⟶CO2+H2O
It can be seen that, there are 12 hydrogen in the left side of the reaction while it is two hydrogen in the right side of the reaction. By multiplying 6 as a coefficient of hydrogen. Hence, the balanced chemical equation will be
C6H12O2+ 8O2⟶CO2+6H2O
The balanced chemical equation is
(i) CaCO3+2HCl⟶CaCl2+CO2+H2O
(ii) C6H12O2+ 8O2⟶CO2+6H2O
To know more about balanced chemical equation
https://brainly.com/question/15052184
#SPJ2
In a thermonuclear device, the pressure of 0.050 liters of gas within the bomb casing reaches 458000 atm. When the bomb casing is destroyed by the explosion, the gas is released into the atmosphere where it reaches a pressure of 1.00 atm. What is the volume of the gas after the explosion
Answers
Answer:
22900 L
Explanation:
Assuming the gas behaves ideally, we can solve this problem by using Boyle's law, which states that:
P₁V₁=P₂V₂Where subscript 1 refers to the initial conditions of pressure and volume (within the bomb, namely), while 2 refers to the final conditions.
Meaning that in this case:
P₁ = 458000 atmV₁ = 0.050 LP₂ = 1.00 atmV₂ = ?We input the data:
458000 atm * 0.050 L = 1.00 atm * V₂And solve for V₂:
V₂ = 22900 LDraw the structure of the product formed when the following compound is heated in aqueous base. The formula for the product is C8H12O.
Answers
Due to the delocalization of pi bonds, benzene has an aromatic structure whereas cyclohexane only has a cyclic structure.
A transparent, colorless liquid with a sweet smell is cyclohexene. It serves as a catalytic solvent, a chemical building block, and an oil extraction process. It can also be detected in motor vehicle exhaust. It belongs to the class of cyclohexanols and is a secondary alcohol. The natural substance cyclohexanol is known to be present in Gossypium hirsutum. The fundamental distinction between cyclohexane and benzene is that the former has six carbon atoms and two hydrogen atoms bonded to each of its six carbon atoms, whilst the latter has six hydrogen atoms attached to each of its six carbon atoms.
Learn more about cyclohexene here-
https://brainly.com/question/6854548
#SPJ4
